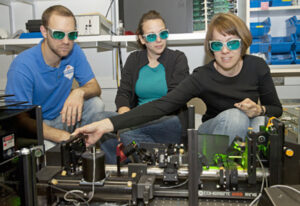
It’s the stuff of great novels, movies, TV quiz shows and rational logic. Sophia Hayes, associate professor of chemistry in Arts & Sciences, faced a tough decision at the dawn of her career.
After finishing an internship at Sandia National Laboratory in 1993, the materials science group there offered her a no-strings-attached grant of about $250,000 to pursue graduate studies at the University of California, Santa Barbara.
There was one sticky stipulation: She had to choose between two professors as her adviser. One was a well-known materials scientist, the other a lesser-known chemist who was doing innovative research in nuclear magnetic resonance (NMR), a field that Hayes found particularly appealing.
Sandia made it clear that, because of a conflict of interest, they could work with one of the professors, but not the other. Thus, everything depended upon “Sophia’s Choice.”
“The Sandia people told me that they didn’t want to influence my choice of research adviser because it would be the most important one I’ll make, second only to whom I would marry,” Hayes says.
Hayes would have gotten a full ride, plus stipend, anyway, but the additional research funds to run an independent project is phenomenal. Hayes felt certain that Sandia was leaning toward the famous materials scientist, but, intellectually, she was drawn to the NMR specialist.
“I agonized for weeks,” she says. “Both professors wanted to know my decision as the deadline approached. At the end, the NMR guy, Hellmut Eckert, told me that he could see it was tearing me up, and he didn’t want me to be so conflicted.
“He said that it would be fine with him if I went with the materials scientist. I thought: ‘Wow, this man is selflessly willing to do this (giving up a huge chunk of funding). That’s it!’
“I got on the phone and told them ‘You probably want the other guy, but it’s got to be Eckert,’ and all this cheering erupted in the background. The conflict would have been with the materials scientist. I was overjoyed.”
Her own spin
That choice heralded Hayes’ extraordinary research saga in NMR, which takes the behavior of nuclear (and electron) spins in magnetic fields and uses them as probes of solid state structure and dynamics and relating these to properties of the system under study.
In medicine, a similar technique is called magnetic resonance imaging (MRI), the test that athletes get to determine injury extent, while NMR is the test that can elucidate enzyme behaviors, polymer and amorphous structures, semiconductor opto-electronics and the behavior and nature of gases and materials for a wide assortment of applications.
At Santa Barbara, Hayes pursued a new wave of NMR research that led to advancements in battery and composite technologies and inspired her to develop her own spin, as it were, on a developing type of NMR, called optically pumped NMR (OPNMR), in which a laser is shined on semiconductor materials held at 6 degrees Kelvin.
Today, she is building Generation III of her technique, which she advanced as a postdoc at Lawrence Livermore National Laboratory and has improved at WUSTL since coming here in 2001.
Semiconductors can conduct electricity under some conditions but not others, making them ideal for controlling electrical current. Silicon is the best-known elemental semiconductor and forms the basis of most integrated circuits in computers. A semiconductor device can perform the function of a vacuum tube — the stuff of old radios and TVs — but the vacuum tube has hundreds of times its volume.
“OPNMR is a great probe of the band structure of a semiconductor, and we are pushing the envelope in detecting the defects at very low concentrations in semiconductors (on the order of 1 in 10,000,000 atoms),” Hayes says.
“If you think of solar energy — solid-state lighting, any kind of photodetection, like a CCD camera — all are based on the process of light hitting a semiconductor that generates electrons or electricity.
“Anything that we can do to improve our understanding of these related phenomena is the aim of our research. We are getting a new laboratory built on the second floor of McMillen Hall with brand new equipment in support of the technique.”
Collaborative science
Another exciting research thrust is a collaborative effort, funded by the university’s Consortium for Clean Coal Utilization, with fellow NMR aficionado Mark Conradi, professor of physics in Arts & Sciences, and Philip Skemer, assistant professor of earth and planetary sciences in Arts & Sciences.
In this work, she uses her technique to study the greenhouse gas carbon dioxide and test possible ways of sequestering, or storing, it in safe environments, such as the ocean.
Theoretically, it’s possible to take carbon dioxide, use chemistry to convert it into a solid, such as cement or a mineral, or catalyze it so it changes into something useful, such as methanol.
“A couple of years ago, I went to Mark, a good friend, and told him with all of his expertise in building hardware and high-pressure NMR and gas diffusion, combined with my inorganic chemistry background, we should watch what CO2 does under pressure and briney conditions such as we’d see in underground CO2 sequestration sites,” Hayes says.
“We are beginning to see some exciting interactions that open up intriguing possibilities. For instance, NMR allows us to actually see the balance of CO2 coming into rock and converting to bicarbonate.
“I couldn’t do this on my own,” she says. “It’s because Phil came on board to figure out the important rocks, and, most importantly, Mark to build high-pressure probes that we’ve made the headway that we have.
“This is what I’d always hoped science would be like, highly collaborative, exactly what attracted me as an undergraduate,” Hayes says.
“I really enjoy the collaboration with Professor Hayes,” Conradi says. “Between Sophia, Phil Skemer and me, there are very different areas of expertise.
“The work on the CO2 project is in the hands of two really talented graduate students from chemistry, Andy Surface and Jeremy Moore. I have enjoyed the collaboration and look forward to extending it.”
Hands-on solving of real-world problems
Hayes, who grew up near Pasadena, Calif., was at the University of California, Berkeley, leaning toward an economics major — her mother’s side of the family had businesses in Japan — when she stumbled into a quantum mechanics class and then a chemistry class with a collaborative research focus.

Research projects were the hook, and “I crammed the chemistry major into my last two years,” Hayes says.
“I’ve encouraged undergraduate research here because of my positive experiences as an undergrad,” she says. “I loved the hands-on solving of real-world problems, where you might try 50 things and only one works. These projects all involved teams of students working with an adviser. I’m so happy to see cross-collaborations all over our campus now.”
Hayes oversees six graduate students and four undergrads in her lab and is involved locally and on-campus with the university’s Institute for School Partnership, the science outreach program for K-12 schools.
In 2011, she joined researchers at the University of Oregon, Oregon State University and Rutgers University to help direct the National Science Foundation’s new Center for Chemical Innovation, a five-year, $20 million program focused on the search for methods to make the next generation of electronic materials.
“NSF identified us as having great NMR instrumentation, which also is a reflection on Mark (Conradi), Jake Schaefer (PhD, the Charles Allen Thomas Professor of Chemistry), Joe Ackerman (PhD, the William Greenleaf Eliot Professor of Chemistry) and others who have made landmark contributions to NMR,” Hayes says.
“I think this center is working hard for technology transfer out of universities into realizable companies and inventions. It’s terrific to be a part of it.”
Fast facts about Sophia Hayes
Family: Husband, Chris Hagedorn (who graduated from WUSTL in 1995 with a bachelor’s degree in chemical engineering), daughter, Marina, 6. Father, Tom, was an engineer; mother, Tatiana, is an accomplished pianist, half-Japanese, half-Russian. Hayes, thus, is one-quarter Japanese.
Hobbies on vacation: scuba diving, sailing
Education: BS, chemistry, 1990, University of California, Berkeley; PhD, chemistry, 1999, University of California, Santa Barbara
Other choices: Hayes worked as an energy/environmental consultant for three years before pursuing a doctorate. She studied traditional Japanese art for a summer, living in a shrine on the outskirts of Kyoto. She played orchestral clarinet (occasionally bassoon) and contemplated music school in lieu of Berkeley.