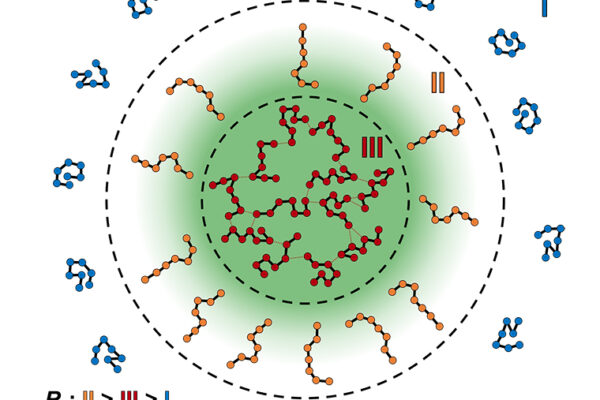
Cells are compartmentalized into distinct communities, with organelles and membranes keeping specific proteins and processes in one place. Interestingly, even without the benefit of a membrane, proteins and molecules can be concentrated into membraneless bodies known as biomolecular condensates.
These condensates include bodies known as stress granules that form and dissolve in response to and the release of cellular stresses. Condensates come together in discrete areas, like a workplace breakout room. Aberrant formation and dissolution of condensates are implicated in a range of human diseases including amyotrophic lateral sclerosis (ALS).
Since condensates are temporary structures, little is known about their organization. Do condensates have organization at all or are they haphazard messes of protein? A group led by Rohit Pappu, the Gene K. Beare Distinguished Professor of Biomedical Engineering at the McKelvey School of Engineering at Washington University in St. Louis, mapped out how condensates formed by prion-like domains (PLDs) of two proteins, hnRNPA1 and FUS, are organized. Mutations in both the PLDs proteins are linked with increased risk of ALS. The research was published in Nature Communications Sept. 8.
Read more on the McKelvey School of Engineering website.