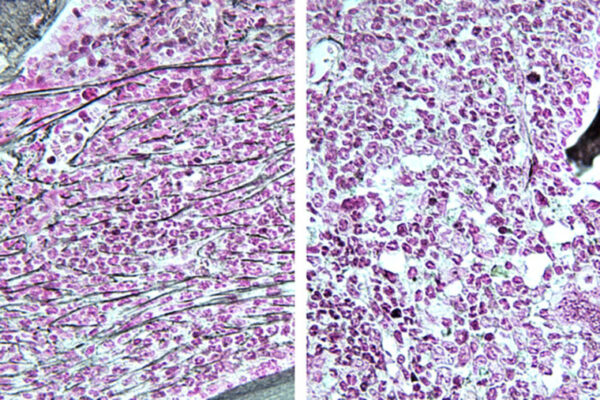
A gene called DNMT3A is important for guiding blood stem cells into forming all the cell types present in blood, including red blood cells, white blood cells and platelets. When this gene accumulates mutations — which might occur with age or due to environmental exposures such as smoking — a person’s risk of developing blood cancers such as acute myeloid leukemia (AML) increases.
DNMT3A operates by adding chemical tags to the DNA molecule in a process called DNA methylation. Until now, DNA methylation was the only known function of this gene, but how altered DNA methylation patterns — caused by mutated versions of DNMT3A — contribute to altered blood cell formation and blood cancer has been unclear.
Now, new research from WashU Medicine investigators provides evidence that DNMT3A has previously unknown ways of influencing blood stem cell longevity and genome stability that are entirely unrelated to its methylation functions. The study, published in the journal Cell Stem Cell, was led by Grant A. Challen, a professor of medicine in the Division of Oncology. Challen is also a research member of Siteman Cancer Center, based at Barnes-Jewish Hospital and WashU Medicine.
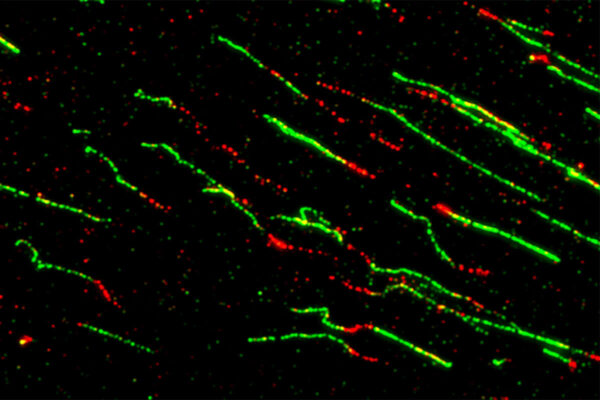
The study, conducted in mouse models and human cells, shows that, independently of methylation, DNMT3A has an impact on the length of telomeres — the endcaps on DNA molecules that shorten with each cell division, limiting the number of divisions it can undergo. When DNMT3A is missing or dysfunctional, telomeres stay long, thus removing replication limits and allowing blood stem cells to multiply indefinitely, a hallmark of cancer. Further experiments also implicated previously unknown roles for this gene in DNA damage response, which also plays roles in cancer development and progression. The discoveries broaden the understanding of this gene’s roles in blood cancers and could help reveal new approaches to treatment.