Picture a living cell as if it were a city. If you were the urban planner for this (very little, very alive) city, one of the things you would have to decide is how to allocate space for different functional uses.
Parts of the city should be zoned for residential use, for example, while others should be zoned industrial; at the same time, you have to put the trash somewhere. Ideally, all of these essential activities should be compartmentalized so they don’t get in the way of the others.

As a city grows, its different compartments also need to grow. This is true in a living cell, too. But do all of the pieces grow at the same rate? How do you assign the resources to grow?
Up until now, scientists have had very little visibility into how the cell organizes and prioritizes the growth of its compartments: the functional structures known as organelles. This includes some of the more familiar organelles like the cell’s nucleus or its energy-making factories of mitochondria.
A new study led by Shankar Mukherji, an assistant professor of physics in Arts & Sciences at Washington University in St. Louis, is one of the first to take a systems approach to measuring and interpreting the changes that occur in organelles as living cells grow. His team conducted their research, published June 6 in Cell Systems using rainbow yeast cells as their study system.
“We used a technology known as hyperspectral imaging, which lets us label virtually all of the major organelles in the cell so that we can directly observe how the cell allocates space to these compartments,” Mukherji said.
Although his team did not invent hyperspectral imaging, Mukherji said, they are among the first handful of researchers to apply it to understanding cell growth.
The new approach allowed them to simultaneously visualize six major metabolically active organelles. Rather than looking at single or pairwise correlations among the organelles, as had been done in the past, the physicists could set up their experiments and then let the data tell them what was happening with all of the organelles at once.
“Using techniques from data science, we could see how the cells patterned themselves in terms of the space for organelles as we changed the conditions they were grown in or as we changed their signaling pathways,” Mukherji said.
Mukherji hopes that the methodology they developed — and the new mathematical theory framework his group constructed as part of this analysis — will help others studying how cells regulate metabolism and growth, which is important in both health and disease.
Making space
The scientists discovered that certain organelles within a cell grow faster than others.
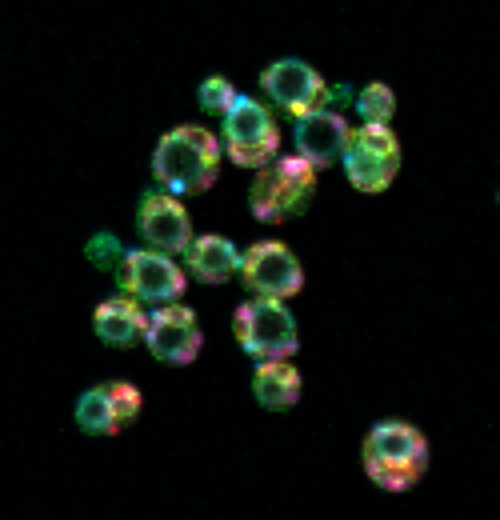
“The cell has to do something intelligent to allow the compartments to grow, but not at the same rate,” Mukherji said. “Basically, it will pick out certain organelles over others, in order to meet increased metabolic demands.”
They also observed that one particular organelle, the vacuole, seems to have an outsized role in helping the cell grow at a steady rate in a constant environment.
“The vacuole seems to be really good at buffering the cell against randomness,” Mukherji said. “At the same time, if the cell actually needs to change its growth rate — because its environment changes or something — then this is the organelle that seems to respond in the right way.
Finally, the researchers took a look at what their data told them about what sets off changes in organelle growth.
“With all the hoops we made the cells jump through, it turns out that the pattern of organelle growth that is triggered by changes in cell size is totally different from the pattern of organelle growth triggered by changes in just growth rate alone,” Mukherji said.
This matters because it could help explain why eukaryotic cells are so flexible in terms of how their sizes and the rates at which they grow and metabolize are related.
“If competing demands from size and growth rate are able to independently be communicated to the cell as a whole, maybe it makes it easier for the system as a whole to balance these competing demands,” he said.
As a next step, Mukherji and his team plan to use these same ideas to characterize human cells. “It’s possible that we’ll find that the normal relationship between a cell’s organelle profile, its size and its growth and metabolic properties become abnormal in disorders that feature abnormal metabolism, from cancer to diabetes to immunological diseases,” he said. “Whether we can uncover these patterns — and whether they are only diagnostic, prognostic or actually underlie disease — is something we’re excited to get working on!”