Wearable or implantable devices to monitor biological activities, such as heart rate, are useful, but they are typically made of metals, silicon, plastic and glass and must be surgically implanted. A research team in the McKelvey School of Engineering at Washington University in St. Louis is developing bioelectronic hydrogels that could one day replace existing devices and have much more flexibility.
Alexandra Rutz, an assistant professor of biomedical engineering, and Anna Goestenkors, a fifth-year doctoral student in Rutz’s lab, created novel granular hydrogels. They are made of microparticles that could be injected into the body, spread over tissues or used to encapsulate cells and tissue and also to monitor and stimulatie biological activity. Results of their research were published Oct. 8 in the nanoscience journal Small.
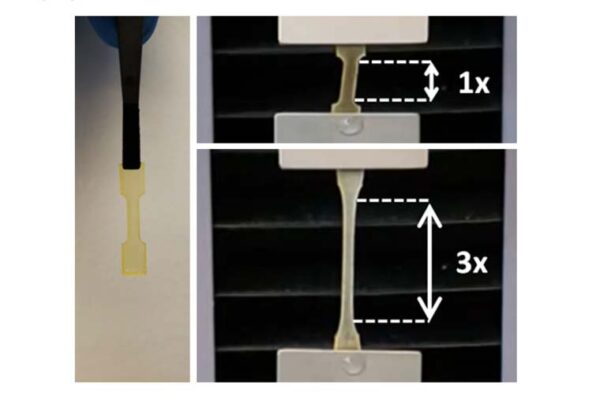
The microparticles are spherical hydrogels made from the conducting polymer known as PEDOT:PSS. When packed tightly, they are similar to wet sand or paste: They hold as a solid with micropores, but they can also be 3D printed or spread into different shapes while maintaining their structure or redistributed into individual microparticles when placed in liquid.
“Granular hydrogels have not been widely studied for these applications, but we have found that this material has the potential to be injected with a needle at the site,” Rutz said. “We’re trying to borrow techniques from tissue engineering to try to have these electronically conducting materials emulate properties of the body while being able to leverage the function of these materials to have more sophisticated ways of doing it.”
When the particles are packed closely together, there are empty spaces between them that create porosity on the micron scale or the cell scale, Goestenkors said.
“Because the particles’ connections aren’t permanent, they can move relative to each other, and the material will flow like a liquid when you apply a certain amount of force that allows them to be injected or extruded,” she said. “But when you remove that force, they recover those connections and become more of a paste-like solid again, so it’s a very adaptable material.”
The individual particles can be pushed through a 3D printing nozzle to form strands, Goestenkors said. She created them through a water-and-oil emulsion, similar to making an oil-and-vinegar salad dressing. After heating the oil, she added the polymer. When stirred, the polymer broke into tiny droplets in the oil, and the elevated temperature crosslinked the polymer to create stable hydrogels.
As part of their research, they conducted an experiment with locusts in the lab of Barani Raman, the Dennis & Barbara Kessler Professor at McKelvey Engineering and co-director of WashU’s Center for Cyborg and BioRobotic Research. Goestenkors put small clumps of the particles on the tips of locust antennae, which have olfactory receptor neurons. The particles allowed them to measure local field potentials that correspond with an odor being sensed by the locust.
“With further development, we envision these conducting granular hydrogels could be used as 3D printed customized electrodes that can conform to topographically diverse surfaces or completely encapsulate biological components, tissue engineering scaffolds or injectable therapies,” Rutz said.
Rutz and Goestenkors have applied for a U.S. patent that covers fabrication and applications of conducting polymer microparticles and conducting granular hydrogels. They are working with WashU’s Office of Technology Management, which is assisting in protecting the intellectual property and advancing commercialization efforts.
Goestenkors AP, Yu JS, Park J, Wu Y, Vargas Espinoza CJ, Friedman LC, Okafor SS, Liu T, Chatterjee S, Debnath A, Semar BA, O’Hare CP, Alvarez RM, Singamaneni S, Raman B, Rutz AL. PEDOT:PSS Microparticles for Extrudable and Bioencapsulating Conducting Granular Hydrogel Bioelectronics. Small. Oct. 8, 2025. DOI: https://doi.org/10.1002/smll.202506438
This research was supported by Washington University in St. Louis through the Women’s Health Technologies Collaboration Initiation Grant, Center for Regenerative Medicine Seed Grant, Ovarian Cancer Research Innovation Fund Award, the McDonnell Center for Cellular and Molecular Neurobiology Small Grant, and the Center for Cyborg and BioRobotic Research.
Originally published on the McKelvey Engineering website