In recent years, research has begun to reveal that the lines of communication between the body’s organs are key regulators of aging. When these lines are open, the body’s organs and systems work well together. But with age, communication lines deteriorate, and organs don’t get the molecular and electrical messages they need to function properly.
A new study from Washington University School of Medicine in St. Louis identifies, in mice, a critical communication pathway connecting the brain and the body’s fat tissue in a feedback loop that appears central to energy production throughout the body. The research suggests that the gradual deterioration of this feedback loop contributes to the increasing health problems that are typical of natural aging.
The study — published Jan. 8 in the journal Cell Metabolism — has implications for developing future interventions that could maintain the feedback loop longer and slow the effects of advancing age.
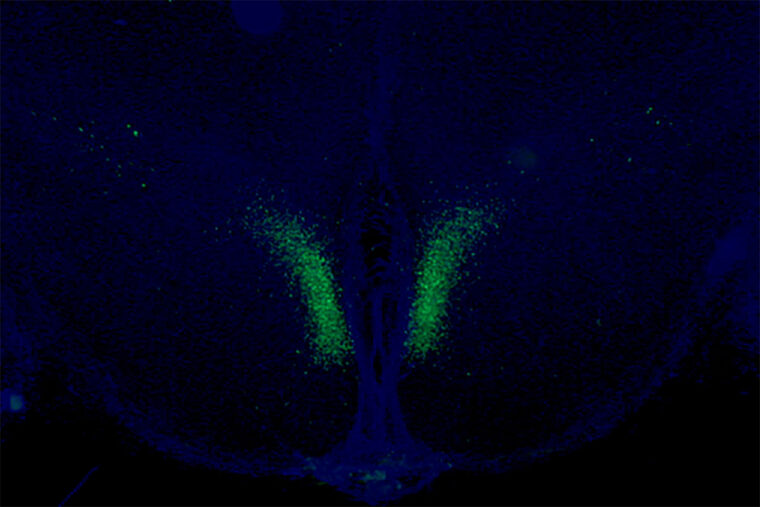
The researchers identified a specific set of neurons in the brain’s hypothalamus that, when active, sends signals to the body’s fat tissue to release energy. Using genetic and molecular methods, the researchers studied mice that were programmed to have this communication pathway constantly open after they reached a certain age. The scientists found that these mice were more physically active, showed signs of delayed aging, and lived longer than mice in which this same communication pathway gradually slowed down as part of normal aging.
“We demonstrated a way to delay aging and extend healthy life spans in mice by manipulating an important part of the brain,” said senior author Shin-ichiro Imai, MD, PhD, the Theodore and Bertha Bryan Distinguished Professor in Environmental Medicine and a professor in the Department of Developmental Biology at Washington University. “Showing this effect in a mammal is an important contribution to the field; past work demonstrating an extension of life span in this way has been conducted in less complex organisms, such as worms and fruit flies.”
These specific neurons, in a part of the brain called the dorsomedial hypothalamus, produce an important protein — Ppp1r17. When this protein is present in the nucleus, the neurons are active and stimulate the sympathetic nervous system, which governs the body’s fight or flight response.
The fight or flight response is well known for having broad effects throughout the body, including causing increased heart rate and slowed digestion. As part of this response, the researchers found that the neurons in the hypothalamus set off a chain of events that triggers neurons that govern white adipose tissue — a type of fat tissue — stored under the skin and in the abdominal area. The activated fat tissue releases fatty acids into the bloodstream that can be used to fuel physical activity. The activated fat tissue also releases another important protein — an enzyme called eNAMPT — which returns to the hypothalamus and allows the brain to produce fuel for its functions.
This feedback loop is critical for fueling the body and the brain, but it slows down over time. With age, the researchers found that the protein Ppp1r17 tends to leave the nucleus of the neurons, and when that happens, the neurons in the hypothalamus send weaker signals. With less use, the nervous system wiring throughout the white adipose tissue gradually retracts, and what was once a dense network of interconnecting nerves becomes sparse. The fat tissues no longer receive as many signals to release fatty acids and eNAMPT, which leads to fat accumulation, weight gain and less energy to fuel the brain and other tissues.
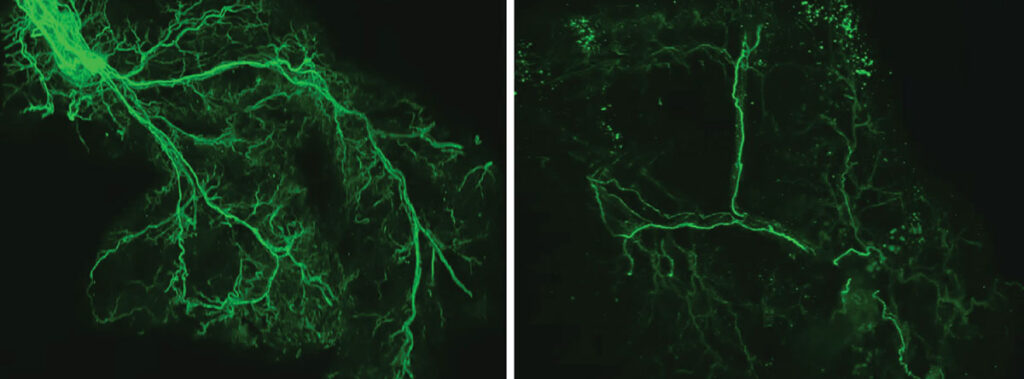
With age, the researchers found that the protein Ppp1r17 tends to leave the nucleus of the neurons, and when that happens, the neurons in the hypothalamus send weaker signals. With less use, the nervous system wiring throughout the white adipose tissue gradually retracts, and what was once a dense network of interconnecting nerves (left) becomes sparse (right). Credit: Kyohei Tokizane
The researchers, including first author Kyohei Tokizane, PhD, a staff scientist and a former postdoctoral researcher in Imai’s lab, found that when they used genetic methods in old mice to keep Ppp1r17 in the nucleus of the neurons in the hypothalamus, the mice were more physically active — with increased wheel-running — and lived longer than control mice. They also used a technique to directly activate these specific neurons in the hypothalamus of old mice, and they observed similar anti-aging effects.
On average, the high end of the life span of a typical laboratory mouse is about 900 to 1,000 days, or about 2.5 years. In this study, all of the control mice that had aged normally died by 1,000 days of age. Those that underwent interventions to maintain the brain-fat tissue feedback loop lived 60 to 70 days longer than control mice. That translates into an increase in life span of about 7%. In people, a 7% increase in a 75-year life span translates to about five more years. The mice receiving the interventions also were more active and looked younger — with thicker and shinier coats — at later ages, suggesting more time with better health as well.
Imai and his team are continuing to investigate ways to maintain the feedback loop between the hypothalamus and the fat tissue. One route they are studying involves supplementing mice with eNAMPT, the enzyme produced by the fat tissue that returns to the brain and fuels the hypothalamus, among other tissues. When released by the fat tissue into the bloodstream, the enzyme is packaged inside compartments called extracellular vesicles, which can be collected and isolated from blood.
“We can envision a possible anti-aging therapy that involves delivering eNAMPT in various ways,” Imai said. “We already have shown that administering eNAMPT in extracellular vesicles increases cellular energy levels in the hypothalamus and extends life span in mice. We look forward to continuing our work investigating ways to maintain this central feedback loop between the brain and the body’s fat tissues in ways that we hope will extend health and life span.”
Tokizane K, Brace CS, Imai S. DMHPpp1r17 neurons regulate aging and lifespan in mice through hypothalamic-adipose inter-tissue communication. Cell Metabolism. Jan. 8, 2024. https://doi.org/10.1016/j.cmet.2023.12.011
This work was supported by the National Institute on Aging of the National Institutes of Health (NIH), grant numbers AG037457 and AG047902; the American Federation for Aging Research; the Tanaka Fund at Washington University School of Medicine; a Glenn Foundation for Medical Research Postdoctoral Fellowship; and a Tanaka Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,800 faculty. Its National Institutes of Health (NIH) research funding portfolio is the third largest among U.S. medical schools, has grown 52% in the last six years, and, together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,800 faculty physicians practicing at 65 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare. WashU Medicine has a storied history in MD/PhD training, recently dedicated $100 million to scholarships and curriculum renewal for its medical students, and is home to top-notch training programs in every medical subspecialty as well as physical therapy, occupational therapy, and audiology and communications sciences.
Originally published on the School of Medicine website