The world’s first nasal vaccine for COVID-19 — based on technology licensed from Washington University in St. Louis — was approved Sept. 6 in India for emergency use. Since the vaccine is delivered via the nose, right where the virus enters the body, it has the potential to block infection and break the cycle of transmission, as well as prevent lung damage. Washington University scientists developed the nasal vaccine in collaboration with Bharat Biotech International Limited in India, a global leader in vaccine innovation and a developer of vaccines for infectious diseases.
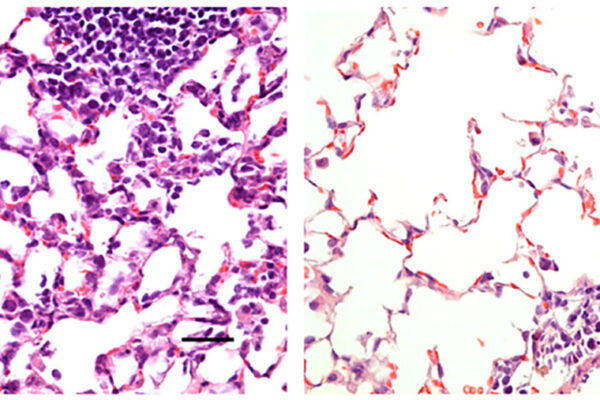
Early studies at Washington University showed that nasal delivery of this vaccine creates a strong immune response throughout the body, especially in the nose and respiratory tract. In animal studies, the nasal vaccine prevented infection from taking hold in the body. While injectable COVID-19 vaccines have been shown to elicit protection against severe disease, this route of administration is thought to be less effective at preventing infection and possibly transmission.
“Nasal vaccines induce the type of protective immunity that we think will prevent or limit infection and also curb pandemic transmission of this virus,” said Michael S. Diamond, MD, PhD, the Herbert S. Gasser Professor of Medicine, a professor of molecular microbiology, and of pathology & immunology, and a co-inventor of the vaccine. “The primary goal of vaccines is to prevent hospitalization and death, of course, but if we can also reduce infection, that would be even better. The more people the virus infects, the more chances it has to spin off new variants, which sustain the pandemic. There are always some people who can’t get vaccinated or who are still at risk of severe illness despite vaccination, and the best way to protect them is to stop the virus from circulating.”
The vaccine was created by Diamond, David T. Curiel, MD, PhD, the Distinguished Professor of Radiation Oncology at Washington University School of Medicine, and members of their laboratories. SARS-CoV-2, the virus that causes COVID-19, uses a spike protein to invade cells. The researchers inserted that spike protein into a virus that causes the common cold, called an adenovirus. But the scientists tweaked the adenovirus, rendering it unable to cause illness. The harmless adenovirus carries the spike protein into the nose, enabling the body to mount an immune defense against the SARS-CoV-2 virus without becoming sick. The new vaccine also incorporates two mutations into the spike protein that stabilize it in a specific shape that is most conducive to forming antibodies against it. Additionally, the researchers genetically modified the adenovirus to improve production of the spike protein. Both innovations were designed to elicit a strong immune response.
After studies in 2020 and 2021 led by Diamond and Curiel demonstrated that the vaccine is effective in mice and nonhuman primates, Bharat Biotech licensed the technology from Washington University, refined the delivery device and scaled up manufacturing. The company launched two clinical trials of the nasal vaccine in India: a phase 3 trial involving about 3,100 previously unvaccinated people who received two doses of the nasal vaccine, and a booster trial with about 875 people who received a single dose of the nasal vaccine after two doses of another COVID-19 vaccine. These trials, which concluded in August, indicated that the vaccine is safe and effective at eliciting a strong immune response in people when used either as a primary vaccine or as a booster.
The nasal delivery system was designed and developed to be cost-effective, a feature that is especially important in low- and middle-income countries, and the vaccine can be stored in a refrigerator. Receiving the vaccine requires only a brief inhalation, a major plus to the many people who prefer to avoid needles. Importantly, the design of the vaccine makes it relatively quick and easy to update when new variants emerge, simply by switching out the current spike protein with one from a new variant. While the vaccine was approved in India for people who are unvaccinated, it eventually may be possible for the vaccine to be used as a booster.
“This pandemic has been dragging on because new variants continue to emerge that are capable of causing a lot of infections and transmission among people who were already vaccinated,” Curiel said. “A nasal vaccine may be what we need to finally break the cycle of transmission. This vaccine, in particular, has been optimized for use in resource-limited settings. I am very excited about the potential of this vaccine to reach the 3 billion people worldwide who are not yet fully vaccinated against COVID-19 and help bring this pandemic to an end.”
Inhaled vaccines may fend off infection better than injected vaccines because they shore up defenses right in the part of the body where the virus is most likely to enter. This week, a COVID-19 vaccine inhaled through the mouth was approved for use in China as a booster. Like the nasal vaccine, this orally inhaled vaccine was designed to enhance immunity in the upper respiratory tract and reduce transmission.
Washington University is exploring licensing the adenoviral nasal vaccine technology in other countries, including the U.S.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,700 faculty. Its National Institutes of Health (NIH) research funding portfolio is the fourth largest among U.S. medical schools, has grown 54% in the last five years, and, together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,790 faculty physicians practicing at over 60 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare. WashU Medicine has a storied history in MD/PhD training, recently dedicated $100 million to scholarships and curriculum renewal for its medical students, and is home to top-notch training programs in every medical subspecialty as well as physical therapy, occupational therapy, and audiology and communications sciences.