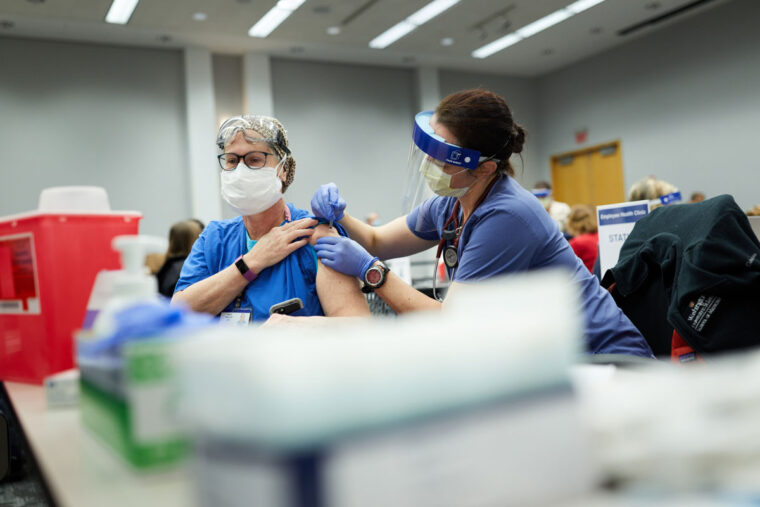
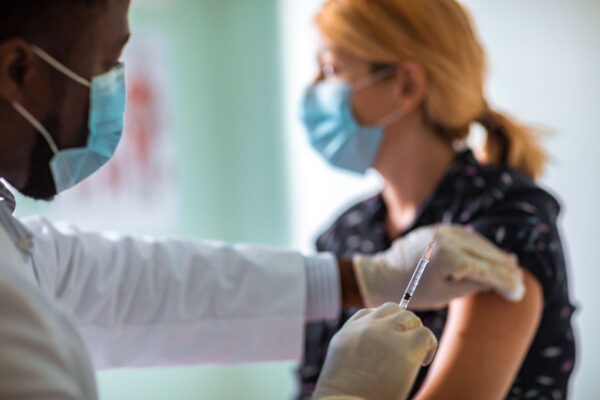
As part of a historic effort to end the COVID-19 pandemic, health-care personnel at Washington University School of Medicine in St. Louis and BJC HealthCare have begun receiving the first doses of a vaccine against the SARS-CoV-2 virus. Almost 10,000 doses of the Pfizer vaccine will be administered in the coming weeks to School of Medicine and BJC employees who have direct contact with patients, work in patient-care areas or handle potentially infectious materials, such as nasal swab samples. Such patient-facing personnel have been prioritized by the Centers for Disease Control and Prevention (CDC) and the state of Missouri to receive the vaccine first.
“We are thankful that it is now possible to offer a safe and effective SARS-CoV-2 vaccine to our front-line personnel,” said David H. Perlmutter, MD, executive vice chancellor for medical affairs, the George and Carol Bauer Dean of the School of Medicine, and the Spencer T. and Ann W. Olin Distinguished Professor. “Our health-care workforce has drawn from a deep well of purpose and perseverance to take care of patients with COVID-19 and allow our hospitals and clinics to provide for the community under the most incredibly trying of circumstances.”
The Food and Drug Administration (FDA) issued an emergency use authorization for the Pfizer/BioNTech vaccine Dec. 11. Vaccinations on the Medical Campus began with a practice run-through Dec. 16, during which five people received the vaccine. The large-scale effort kicked off Dec. 17, with some 420 people receiving the vaccine. BJC will continue to receive additional Pfizer vaccine orders in the coming weeks. The vaccine requires two doses, with the second dose given 21 days after the first.
BJC HealthCare is among the first health-care providers in Missouri and the bi-state region to receive shipments of the vaccine designated for patient-facing workers. However, the first shipment of vaccines will not cover all employees. Because the risk of severe COVID-19 increases with age, vaccine recipients will be prioritized by age, with older groups receiving the vaccine earlier than their younger counterparts.
Prioritized for the first round of vaccines are front-line personnel who work with patients or in close proximity to patients, even if they don’t provide patient care. For example, food-service workers who deliver meals to patients, staff who clean patients’ rooms, and lab personnel who handle patient samples will be as likely as doctors, nurses or respiratory therapists to receive the first round of vaccines.

“With the arrival of the vaccine this week, and the promise of others on the horizon, we’re witnessing the beginning of the end to this pandemic,” said Hilary M. Babcock, MD, professor of medicine and medical director of the BJC Infection Prevention and Epidemiology Consortium. “This a historic moment that brings us hope and makes me deeply grateful for the scientists who have worked so diligently to develop these vaccines, the public health professionals working to get the vaccines distributed, and the health-care workers who’ve fought so hard to take care of so many of us.
“The CDC, their advisory committees and the states developed guidelines to prioritize how to administer these vaccines so it is done accessibly, equitably and fairly,” she said. “After high-priority personnel of all ages have been offered the opportunity to be vaccinated, we will move to other BJC or WUSM staff who work on the Medical Campus but do not, as part of their jobs, come into regular contact with patients or infectious material. As of now, staff able to work remotely will not be offered vaccine until later in the state’s distribution plan.”
The excitement was palpable early Dec. 17 as health-care workers gathered around registered nurse Jennifer Reneau, manager of occupational health at the School of Medicine, to go over steps on how to give the vaccine. “This,” Reneau told the group, “is what hope looks like.”
Among those to receive the vaccine Dec. 17 was Tyrone Simpkins, a sergeant with BJC security. Receiving the shot brought forth a swell of emotions for him.
“I’ve lost 12 family members to COVID this year,” Simpkins said. “I’m going to stop it in my family. … Masks are great, but we’ve got to do something else, and now that we’ve got the vaccine, everybody should be trying to get it.”
Critical care physician Tiffany Osborn, MD, professor of surgery and of emergency medicine, cheered after receiving her shot. “I am so excited about this vaccine being available,” said Osborn, who treats patients at Barnes-Jewish Hospital. “I am so happy that we had this opportunity. This means so much for front-line workers. … Just knowing that you have that extra protection is such a godsend.”
Joan Niehoff, MD, associate professor of anesthesiology, said that after months of being afraid of the virus and watching people suffer through illness, she felt euphoric after receiving the vaccine. “When I got the shot, I cried,” said Niehoff, who treats patients at St. Louis Children’s Hospital.
“I got the vaccine to protect myself and to protect the community,” she explained. “This is the only way that we’re going to get this pandemic to end. This is the only way. … Thank God for science.”

Vaccination is strongly encouraged but not required. Washington University held a virtual town hall Dec. 9 to provide information to employees about the vaccine and the distribution process and to answer questions. A recording of the town hall is available here. A second virtual town hall is scheduled for Dec. 21. BJC HealthCare held a town hall for its employees Dec. 10.
BJC will receive and begin to administer additional vaccines as they become available following new emergency use authorizations from the FDA. Barnes-Jewish Hospital has been designated to receive and distribute the Pfizer vaccines by the state of Missouri because of the hospital’s large ultra-cold storage capacity. The Pfizer vaccines must be kept at sub-zero temperatures. The hospital will receive initial shipments and redistribute the vaccines to other BJC hospitals in the region.
The safety and effectiveness of the Pfizer vaccine was investigated in a phase 3 clinical trial of 43,000 people across many age groups and of diverse ethnic and racial backgrounds, including 20,000 people over age 56 and 12,900 people of color. The vaccine was found to be 95% effective in preventing COVID-19. There were 162 positive cases in the placebo group, nine of which required hospitalization, compared with eight positive cases in the vaccine group, one of which required hospitalization — a difference that demonstrates a statistically significant 95% reduction in symptomatic infections.
The vaccine’s most common side effects were headache and fatigue, though some people have developed fever and muscle aches, more frequently after the second vaccine dose. There have been rare reports of allergic reactions to the Pfizer vaccine, and as a precaution, staff administering the shots will be prepared to treat such reactions should they occur.
“To ultimately be able to control this viral pandemic, vaccination is critical,” said William G. Powderly, MD, the J. William Campbell Professor of Medicine, the Larry J. Shapiro Director of the Institute for Public Health and director of the Institute of Clinical and Translational Sciences. “That’s why so much emphasis has been placed on developing vaccines as rapidly as possible without cutting corners on safety or efficacy. The data on safety and efficacy are excellent. Our first priority is to get as many people vaccinated as possible, making sure everyone goes back for that second dose. The second dose is critical to creating a sustained, protective immune response.”
According to Babcock, Powderly and their colleagues, those who have been vaccinated must continue wearing masks and social distancing. The data demonstrate that the Pfizer vaccine prevents illness, but whether it prevents a vaccinated person from spreading the virus is still under investigation.
The Pfizer vaccine does not contain the SARS-CoV-2 virus, not even in a weakened or inactive form. Rather, the vaccine delivers a molecule called mRNA that instructs human cells to manufacture the virus’ spike protein. In this way, the immune system gets a safe look at the spike protein, which coats the surface of the real SARS-CoV-2. The body then can mount an immune response to the foreign protein without any chance of infection. The second dose solidifies the immune system’s memory of the spike protein. Later, if the body ever encounters the real virus, it will recognize the spike protein, and the immune system will trigger a fast and effective attack on the virus.
“The Pfizer vaccine data show safety and effectiveness that is equivalent to or better than most commonly used vaccines, such as those given to prevent shingles and measles, mumps and rubella,” said Rachel M. Presti, MD, PhD, associate professor of medicine and medical director of the Infectious Diseases Clinical Research Unit. “We offer our most sincere thanks to the thousands of research volunteers and scientific staff who have made these vaccines possible.”
Elizabethe Holland Durando contributed to this report.