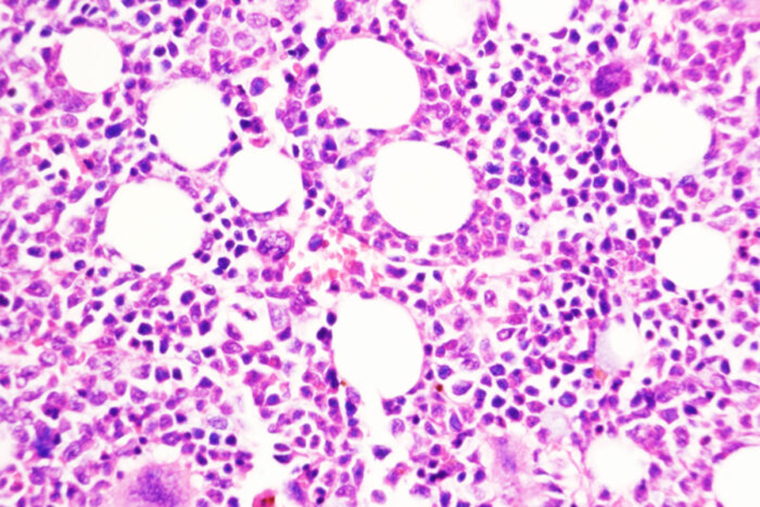
A type of chronic leukemia can simmer for many years. Some patients may need treatment to manage this type of blood cancer — called myeloproliferative neoplasms (MPN) — while others may go through long periods of watchful waiting. But for a small percentage of patients, the slower paced disease can transform into an aggressive cancer, called secondary acute myeloid leukemia, that has few effective treatment options. Little has been known about how this transformation takes place.
But now, researchers at Washington University School of Medicine in St. Louis have identified an important transition point in the shift from chronic to aggressive leukemia. They have shown that blocking a key molecule in the transition pathway prevents this dangerous disease progression in mice with models of the disease and in mice with tumors sampled from human patients.
The research appears Dec. 29 in the journal Nature Cancer.
“Secondary acute myeloid leukemia has a grim prognosis,” said senior author Stephen T. Oh, MD, PhD, an associate professor of medicine and co-director of the Division of Hematology at the School of Medicine. “Almost every patient who develops acute leukemia after a history of myeloproliferative neoplasms will die from the disease. Therefore, a major focus of our research is to better understand this conversion from chronic to aggressive disease and to develop better therapies and, hopefully, prevention strategies for these patients.”
The study suggests that inhibiting this key transition molecule — called DUSP6 — helps overcome the resistance that these cancers often develop to JAK2 inhibitors, the therapy typically used to treat them. JAK2 inhibitors are an anti-inflammatory therapy also used to treat rheumatoid arthritis.
“These patients are commonly treated with JAK2 inhibitors, but their disease progresses despite that therapy, so we’re also trying to identify how the disease is able to worsen even in the setting of JAK2 inhibition,” said Oh, who treats patients at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine.
The researchers conducted a deep dive into the genetics of these tumors, both during the slow chronic phase and after the disease had transformed into the aggressive form while patients were taking JAK2 inhibitors. The DUSP6 gene stood out as highly expressed in the 40 patients whose tumors were analyzed in this study.
Using genetic techniques to delete the DUSP6 gene prevented the transition to aggressive disease in mice with models of this cancer. The researchers also tested a drug compound that inhibits DUSP6 and found that the compound — only available for animal research — stopped progression of the chronic disease to the aggressive disease in two different mouse models of the cancer and in mice with human tumors sampled from patients. Reducing DUSP6 levels both genetically and with a drug also reduced inflammation in these models.
Since the drug that inhibits DUSP6 is not available for human clinical trials, Oh and his colleagues are interested in exploring treatments that inhibit another molecule that they found is activated downstream of DUSP6 and that they showed is also required to perpetuate the negative effects of DUSP6. There are drugs in clinical trials that inhibit this downstream molecule, known as RSK1. Oh’s team is interested in investigating these drugs for their potential to block the dangerous transition from chronic to aggressive disease and address resistance to JAK2 inhibition.
“A future clinical trial might enroll myeloproliferative neoplasm patients who are taking JAK2 inhibitors and, despite that, show evidence of their disease worsening,” Oh said. “At that point, we might add the type of RSK inhibitor that’s now in trials to their therapy to see if that helps block progression of the disease into an aggressive secondary acute myeloid leukemia. A newly developed RKS inhibitor is in phase 1 clinical trials for patients with breast cancer, so we’re hopeful our work provides a promising foundation for developing a new treatment strategy for patients with this chronic blood cancer.”
This work was supported by the National Institutes of Health (NIH), grant numbers R01HL134952, T32HL007088 and R01HL147978; the Leukemia and Lymphoma Society Translational Research Program; the MPN Research Foundation; the When Everyone Survives Foundation; the Edward P. Evans Foundation; the Gabrielle’s Angel Foundation; the Leukemia and Lymphoma Society; a Canderel Rising Star Summer Studentship; a Canadian Research Chair in Functional Genomics; and Canadian Institutes of Health Research (CIHR) grants PJT-156233 and PJT-438303. Technical support was provided by the Alvin J. Siteman Cancer Center Tissue Procurement Core Facility; the Biostatistics Shared Resource; the Flow Cytometry Core; Barnes-Jewish Hospital; the Institute of Clinical and Translational Sciences; and the Immunomonitoring Laboratory, which are supported by NCATS Clinical and Translational Sciences Award UL1 TR002345 and National Cancer Institute (NCI) Cancer Center Support Grant P30CA91842. Additional support was provided by the Barnard Cancer Institute. The Immunomonitoring Laboratory is also supported by the Andrew M. and Jane M. Bursky Center for Human Immunology and Immunotherapy Programs.
Kong T, et al. DUSP6 mediates resistance to JAK2 inhibition and drives leukemic progression. Nature Cancer. Dec. 29, 2022.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,700 faculty. Its National Institutes of Health (NIH) research funding portfolio is the fourth largest among U.S. medical schools, has grown 54% in the last five years, and, together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,790 faculty physicians practicing at over 60 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare. WashU Medicine has a storied history in MD/PhD training, recently dedicated $100 million to scholarships and curriculum renewal for its medical students, and is home to top-notch training programs in every medical subspecialty as well as physical therapy, occupational therapy, and audiology and communications sciences.