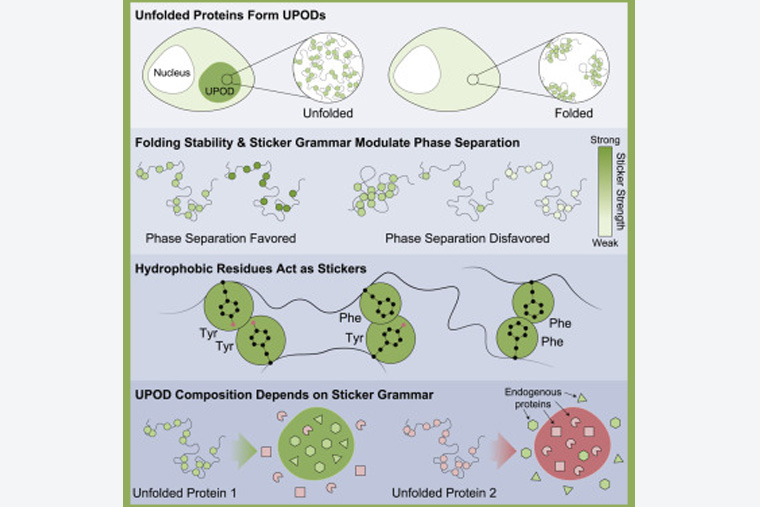
Unfolded proteins are unhealthy proteins. When found inside of cells, they are rounded up, identified, and destroyed. This is an important quality-control process, especially in the brain and the heart.
How these unfolded proteins are identified, however, has been a mystery. Now, research led by Kiersten Ruff, a senior research scientist in the lab of Rohit Pappu, the Gene K. Beare Distinguished Professor of biomedical engineering at Washington University in St. Louis’ McKelvey School of Engineering, has uncovered the rules that govern this process — and found that exceptions to these rules may play a role in dysfunctional cells.

The research was published in the journal Molecular Cell and was a collaborative effort between the labs of Pappu and Danny Hatters, professor of biochemistry at the University of Melbourne in Australia.
A healthy cell will begin cleaning out unfolded proteins once they have collected in clumps called, appropriately, unfolded protein deposits (UPODS). But researchers were not sure how these UPODs formed.

Ruff, Pappu said, came up with an idea.
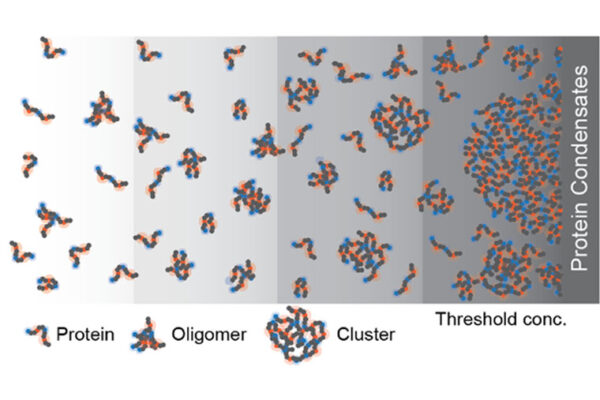
“She had the brilliant insight that there likely was a combination of factors involved in driving the formation of UPODs,” Pappu said. “She reasoned that it could not just be unfolded proteins crossing some threshold concentration. There must be specific interactions that must be mediating UPOD formation.”
Ruff’s insight, driven by a close collaboration with members of the Hatters lab, led to the finding that the formation of UPODs requires the crossing of a threshold concentration in cells along with the presence of specific stickers — particular residues within proteins that drive them to stick to one another.
The team went on to discover that UPODs also become collection grounds for other proteins that have similar sticker chemistries. “UPODS seem to not only attract components of the quality-control machinery, which one would expect, but they are also attractors for other proteins that have similar chemistries,” Pappu said.
They can drag perfectly healthy proteins into what is, effectively, a trash pile. The finding may shed light on the progression of degenerative disorders in cells that do not turn over — such as neurons in diseases including amyotrophic lateral sclerosis, or ALS, or cardiac cells in cardiomyopathies, or heart muscle diseases.
Although the finding relied on high-tech experimentation and careful analysis, Pappu said, “Kiersten’s brilliance and novel insights transformed the nature of the investigations.”
This work was supported by grants APP1161803 and APP1154352 from the National Health and Medical Research Council of Australia, grant DP170103093 from the Australian Research Council, the US National Institutes of Health (5R01NS056114), the US Air Force Office of Scientific Research (FA9550-20-1-0241), and the Wellcome Trust (204963).
The McKelvey School of Engineering at Washington University in St. Louis promotes independent inquiry and education with an emphasis on scientific excellence, innovation and collaboration without boundaries. McKelvey Engineering has top-ranked research and graduate programs across departments, particularly in biomedical engineering, environmental engineering and computing, and has one of the most selective undergraduate programs in the country. With 140 full-time faculty, 1,387 undergraduate students, 1,448 graduate students and 21,000 living alumni, we are working to solve some of society’s greatest challenges; to prepare students to become leaders and innovate throughout their careers; and to be a catalyst of economic development for the St. Louis region and beyond.