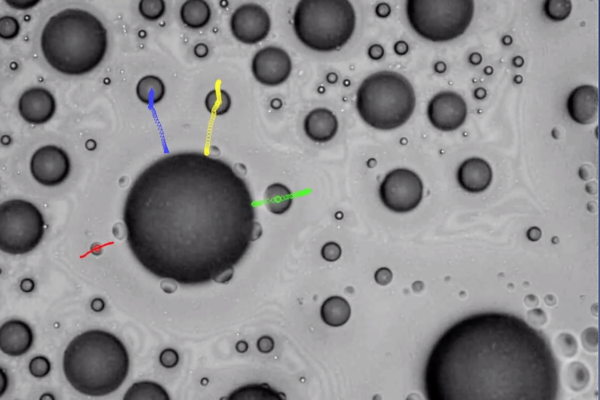
When drops of water touch the surface of a lotus flower leaf, they form beads and roll off, collecting dust particles along the way. In contrast, water droplets on a rose petal also form beads, but remain pinned to the petal’s surface. A mechanical engineer at Washington University in St. Louis combined the two concepts to find a more efficient way for droplets to evaporate from a surface.

Patricia Weisensee, assistant professor of mechanical engineering & materials science in the McKelvey School of Engineering, initially planned to establish a pattern on a surface that would both repel liquid, similar to the lotus leaf, or pin droplets, similar to the rose petal, to influence wetting during droplet impact, such as during rain. Like the lotus leaf, when water impacts a repellent — or superhydrophobic — surface, droplets easily rebound, similar to rain on treated windshields.

In heat transfer and evaporation, these superhydrophobic surfaces are very inefficient due to a short contact time between the water and the surface. Conversely, when liquid comes in contact with a hydrophilic surface that can be wetted, it spreads over the surface, forms a liquid puddle and takes a long time to evaporate. Weisensee wanted to create a surface with both repelling and wetting properties that would create small sub-droplets, combining the advantages of both types of surfaces: droplet pinning and evaporation on the wetting surface without the risk of flooding the entire repelling surface. She then observed their behavior to learn more about evaporation as a cooling method for thermal management of high-tech electronic devices.
Results of her work were published online Dec. 20 in Langmuir.
Read more on the engineering website.