Although some recent studies have suggested that inflammation promotes retinal damage in age-related macular degeneration (AMD), new work from Washington University ophthalmology researchers has found that a particular type of inflammation, regulated by cells called macrophages, actually protects the eye from damage due to AMD.
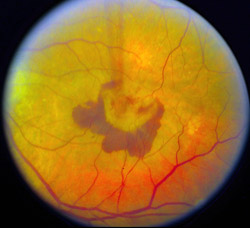
The researchers report in the Aug. 15 issue of Public Library of Science (PLoS) Medicine that in a mouse model of AMD, macrophages help prevent the formation of blood vessels that grow underneath the retina and cause the majority of severe vision loss associated with AMD.
Age-related macular degeneration is the leading cause of blindness in the United States in people over the age of 50. It accounts for more than 40 percent of blindness among the institutionalized elderly, and as baby boomers get older, the problem is expected to grow, with at least 8 million cases of AMD predicted by the year 2020.
There are two varieties of AMD: a “dry” form and a “wet” form. Most patients have the dry form of the disease, and although this can progress and cause severe vision loss in some, between 80 and 90 percent of the blindness and severe vision loss occurs in the wet form of the disease, according to the paper’s first author Rajendra S. Apte, M.D., Ph.D.
“In the wet form of macular degeneration, damaging blood vessels form underneath the retina,” says Apte, assistant professor of ophthalmology and visual sciences at Washington University School of Medicine. “In this study, we looked at some of the mechanisms that control the formation and progression of those vessels.”

The blood vessels are not like the mature vessels in most of the body. Vessels associated with AMD don’t have normal, tight junctions, so they leak and bleed. These blood vessels are located beneath the macula, the center of the retina, and when blood vessels bleed in that location, it leads to vision loss.
Apte says the immune system clearly can play a role in the development of new blood vessels, but there has been conflicting evidence regarding the immune system’s involvement with the damaging vessels made in the wet form of AMD. The most popular idea has been that macrophages and inflammation contribute to the formation of new vessels, but the current study would argue macrophages play the opposite role and that macrophage function is more complex than had been realized.
The research team studied mice whose eyes were treated with a laser that spurs the growth of the damaging vessels. Although the acute laser injury is not identical to the chronic damage from AMD, Apte says the animal model has been remarkably successful in identifying therapies that have proven to be effective in treating AMD.
Apte and co-investigator Thomas A. Ferguson, Ph.D., associate professor of ophthalmology and visual sciences and of pathology and immunology, found that eliminating an anti-inflammatory protein called interleukin-10 (IL-10) decreased growth and development of new blood vessels beneath the retina. Lack of IL-10 led to inflammation and increased the number of macrophages in the eye.

“There were particularly high numbers of macrophages in the eyes of mice without IL-10,” says Ferguson, “but rather than promoting the formation of damaging vessels, the macrophages seem to have prevented them.”
Ferguson and Apte think that as people age, they may experience changes in their production of IL-10, or perhaps the macrophages in their bodies become less efficient over time. Factors such as smoking, uncontrolled high blood pressure or a genetic pre-disposition may enhance the disease process.
“We believe this system involving macrophages and IL-10 provides us with potential targets for therapies that might slow or even reverse the formation of these damaging blood vessels in age-related macular degeneration,” Apte says.
In future studies, they hope to learn more about how these macrophages are working and whether there are specific subtypes of macrophage that play different roles in promoting or inhibiting the blood vessel growth that occurs in AMD.
“Very soon, we’re hoping to look at patients who have macular degeneration and analyze their DNA to see whether they might have abnormalities in their IL-10 gene,” Apte says. “It’s not the only factor involved in vision loss, but our studies indicate that it may be very important.”
Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PloS Medicine, vol. 3:8, Aug. 15, 2006. The paper is available online at http://dx.doi.org/10.1371/journal.pmed.0030310
This research was supported by grants and awards from The National Eye Institute, the Carl Marshall Reeves and Mildred Almen Reeves Foundation, American Federation for Aging Research, Diabetes Research & Training Center Pilot Study & Feasibility Grant and Research to Prevent Blindness.
Washington University School of Medicine’s full-time and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.