Doctors have long known that children who become seriously ill with certain respiratory viruses such as respiratory syncytial virus (RSV) are at elevated risk of developing asthma later in life. What they haven’t known is why.
A new study by researchers at Washington University School of Medicine in St. Louis may have solved the mystery. The study, in mice, shows that respiratory viruses can hide out in immune cells in the lungs long after the initial symptoms of an infection have resolved, creating a persistently inflammatory environment that promotes the development of lung disease. Further, they showed that eliminating the infected cells reduces signs of chronic lung damage before they progress to a full-blown chronic respiratory illness.
The findings, published Oct. 2 in Nature Microbiology, point to a potential new approach to preventing asthma, chronic obstructive pulmonary disease (COPD) and other chronic lung diseases by eradicating the persistent respiratory viruses that fuel these conditions.
“Right now, children who have been hospitalized for a respiratory infection such as RSV are sent home once their symptoms resolve,” said senior author Carolina B. López, a professor of molecular microbiology and a BJC Investigator at WashU Medicine. “To reduce the risk that these children will go on to develop asthma, maybe in the future we will be able to check if all of the virus is truly gone from the lung, and eliminate all lingering virus, before we send them home.”
About 27 million people in the U.S. are living with asthma. Many factors influence a person’s likelihood of developing the chronic breathing illness, including living in a neighborhood with poor air quality, having exposure to cigarette smoke and being hospitalized for viral pneumonia or bronchitis while young. Some researchers — López included — suspected that the link between serious lung infection and subsequent asthma diagnosis was due to lingering virus in the lungs that causes ongoing damage, but a direct link between the ongoing presence of virus and chronic lung disease has not been previously shown.
López and first author Ítalo Araújo Castro, a postdoctoral researcher in her lab, developed a unique system involving a natural mouse virus known as Sendai virus, and fluorescent markers of infection. Sendai is related to human parainfluenza virus, a common respiratory virus that, like RSV, has been linked to asthma in children. Sendai behaves in mice in very much the same way that human parainfluenza virus behaves in people, making it an excellent model of the kinds of infections that could lead to chronic lung disease.
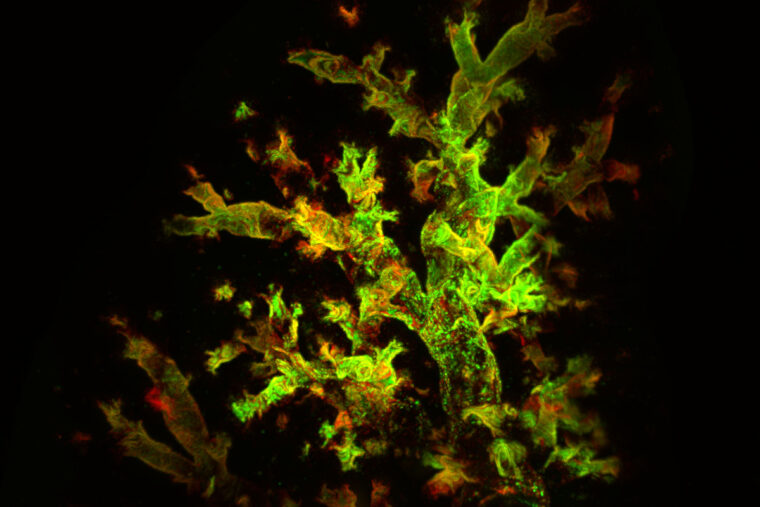
Using the fluorescent trackers, the researchers could observe signs of the virus throughout infection. After about two weeks, the mice recovered, but viral RNA and protein were still detectable several weeks later in their lungs, hidden away in immune cells.
“Finding persistent virus in immune cells was unexpected,” López said. “I think that’s why it had been missed before. Everyone had been looking for viral products in the epithelial cells that line the surface of the respiratory system, because that’s where these viruses primarily replicate. But they were in the immune cells.”
Moreover, the presence of the virus changed the behavior of the infected immune cells, causing them to become more inflammatory than the uninfected immune cells. Persistent inflammation sets the stage for chronic lung disease to arise, the researchers said. Indeed, seven weeks after infection, the mice’s lungs exhibited inflammation of air sacs and blood vessels, abnormal development of lung cells and excess immune tissue — all signs of chronic inflammatory lung damage, even though the mice appeared outwardly to have recovered. Once the infected immune cells were eliminated, the signs of damage diminished.
“We use a perfectly matched virus-host pairing to prove that a common respiratory virus can be maintained in immunocompetent hosts for way longer than the acute phase of the infection, and that this viral persistence can result in chronic lung conditions,” Castro said. “Probably the long-term health effects we see in people who are supposed to be recovered from an acute infection are actually due to persistence of virus in their lungs.”
The findings point to new ways to think about preventing chronic lung diseases, the researchers said.
“Pretty much every single child gets infected with these viruses before the age of 3, and maybe 5% get serious enough disease that they could potentially develop persistent infection,” López said. “We’re not going to be able to prevent children from getting infected in the first place. But if we understand how these viruses persist and the effects that persistence has on the lungs, we may be able to reduce the risk of serious long-term problems.”
Castro IA, Yang Y, Gnazzo V, Kim DH, Van Dyken SJ, López CB. Murine parainfluenza virus persists in lung innate immune cells sustaining chronic lung pathology. Nature Microbiology. Oct. 2, 2024. DOI: 10.1038/s41564-024-01805-8
This project was funded by the National Institutes of Health (NIH), grant numbers AI127832, A137062, HL148033, AI176660 and AI163640; the Washington University BJC Investigators program; and the National Research Foundation of Korea, award number NRF-2020 R1A6A3A03037855. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,900 faculty. Its National Institutes of Health (NIH) research funding portfolio is the second largest among U.S. medical schools and has grown 56% in the last seven years. Together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,900 faculty physicians practicing at 130 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare. WashU Medicine has a storied history in MD/PhD training, recently dedicated $100 million to scholarships and curriculum renewal for its medical students, and is home to top-notch training programs in every medical subspecialty as well as physical therapy, occupational therapy, and audiology and communications sciences.
Originally published on the School of Medicine website