Fractured sleep, daytime sleepiness and other signs of disturbance in one’s circadian rhythm are common complaints of people with Alzheimer’s disease, and the problems only get worse as the disease progresses. But the reason for the link between Alzheimer’s and circadian dysfunction is not well understood.
Researchers at Washington University School of Medicine in St. Louis say that a clue may lie in the brain protein YKL-40. In a study published Dec. 16 in Science Translational Medicine, the researchers report that YKL-40 is both regulated by clock genes and involved in clearing away potentially toxic buildup of Alzheimer’s proteins in the brain. Moreover, Alzheimer’s patients who carry a genetic variant that reduces YKL-40 levels maintain their cognitive faculties longer than people without the variant, the scientists found.
The findings suggest that YKL-40 is a possible link between circadian rhythm dysfunction and Alzheimer’s, and that therapies targeting the protein may slow the course of the disease.
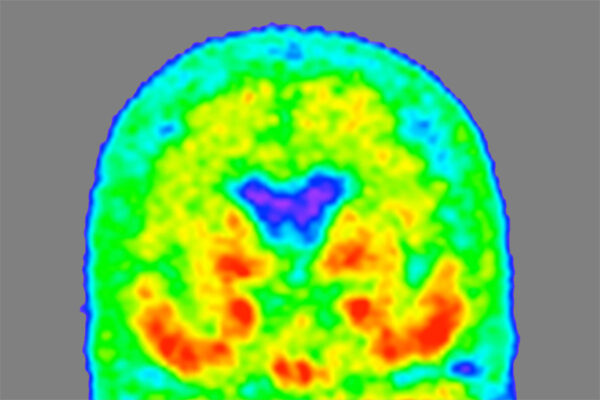
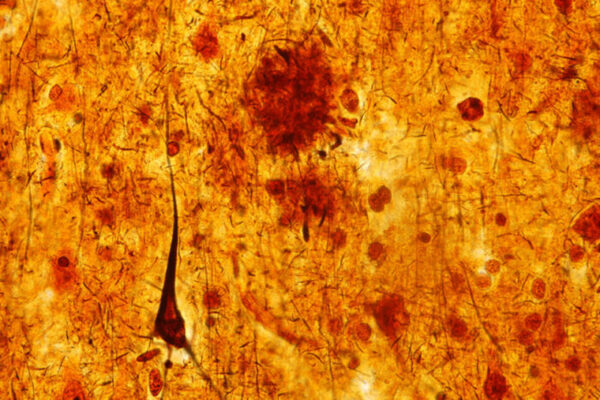
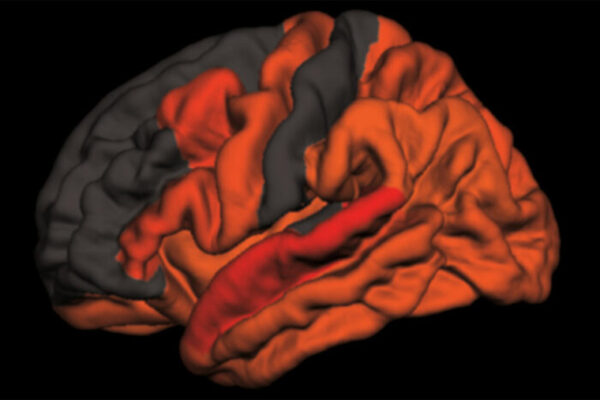
“If your circadian clock is not quite right for years and years — you routinely suffer from disrupted sleep at night and napping during the day — the cumulative effect of chronic dysregulation could influence inflammatory pathways such that you accumulate more amyloid plaques,” said senior author Erik Musiek, MD, PhD, associate professor of neurology. Amyloid plaques in the brain are one of the early hallmarks of Alzheimer’s disease. “We hope that a better understanding of how the circadian clock affects YKL-40 could lead to a new strategy for reducing amyloid in the brain.”
Our daily rhythms are set by a master clock in the brain that is driven by the day and night cycle. Each cell also maintains its own internal clock, pegged to the master clock. A surprisingly broad array of biological processes — from sugar absorption to body temperature to immune and inflammatory responses — vary by time of day.
Although circadian dysfunction affects many aspects of health and disease, it is most easily detected as sleep disturbances, such as difficulty falling asleep or staying asleep at night and increased sleepiness during the day. Such problems are common in people with Alzheimer’s, even those in the earliest stage of the disease, when amyloid plaques have begun forming but cognitive symptoms have not yet appeared.
Musiek, whose work has long focused on the link between circadian rhythm and neurodegenerative diseases such as Alzheimer’s, was conducting a screen for genes regulated by the circadian clock when one specific gene caught his eye.
“The gene for YKL-40 came up as highly regulated by clock genes,” Musiek said. “That was really interesting because it is a well-known biomarker for Alzheimer’s.”
About a decade ago, David Holtzman, MD, the Andrew B. and Gretchen P. Jones Professor and head of the Department of Neurology, and Anne Fagan, professor of neurology, discovered that high levels of YKL-40 in the cerebrospinal fluid are a sign of Alzheimer’s. Subsequent research by Fagan and others revealed that YKL-40 levels rise with normal aging and as Alzheimer’s progresses.
Musiek, first author Brian V. Lananna, then a graduate student, and colleagues set out to explore the connection between the circadian clock, YKL-40 and Alzheimer’s. The disease is characterized by chronic inflammation, so the researchers investigated how the presence or absence of a key circadian gene affects non-neuronal brain cells under inflammatory conditions. They discovered that the clock dictates how much YKL-40 is made.
“If you have inflammation in the morning, you might get lots of YKL-40; if you get inflammation in the evening, when the clock’s in a different phase, you might get less YKL-40,” Musiek said.
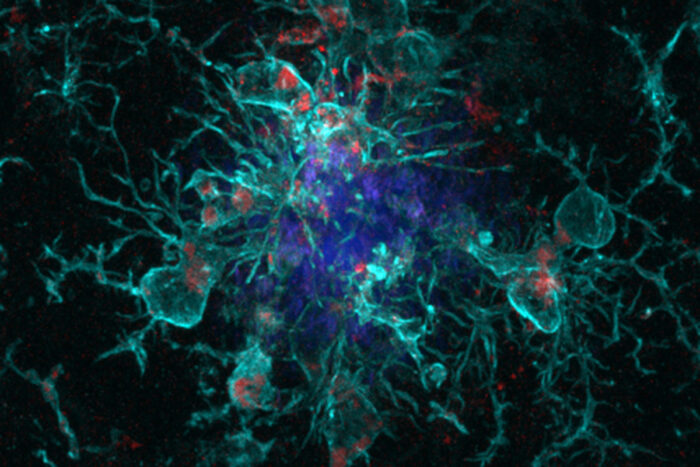
Then, they took Alzheimer’s mouse models prone to developing amyloid plaques and crossed them with genetically modified mice that lack the gene for YKL-40, or with unmodified mice for comparison. Once the mice were eight months old — elderly, by mouse standards — the researchers examined the animals’ brains. The amyloid-prone mice without YKL-40 had about half as much amyloid as those that carried the gene. Amyloid plaques normally are surrounded by immune cells called microglia that help keep the plaques from spreading. In the mice that lacked YKL-40, the microglia were more plentiful and more primed to consume and remove amyloid.
“This YKL-40 protein probably serves as a modulator of the level of microglial activation in the brain,” Musiek said. “When you get rid of the protein, it appears the microglia are more activated to eat up the amyloid. It’s a subtle thing, a tweak in the system, but it seems to be enough to substantially reduce the total amyloid burden.”
This jibes with data from studies in people. Co-author Carlos Cruchaga, professor of psychiatry, of genetics and of neurology, analyzed genetic data from 778 people participating in aging and dementia studies at the university’s Charles F. and Joanne Knight Alzheimer’s Disease Research Center. About a quarter (26%) of them carried a genetic variant that reduces levels of YKL-40. Cognitive skills declined 16% more slowly in the people with the variant.
“People have been measuring YKL-40 in spinal fluid for several years, but we were never sure of its function, if it was good or bad,” Musiek said. “Our data suggest that in Alzheimer’s, it’s bad. People who have less of it fare better. If you could design a therapy to lower YKL-40, it might help the microglia remove more amyloid and maybe slow the progression of disease.”