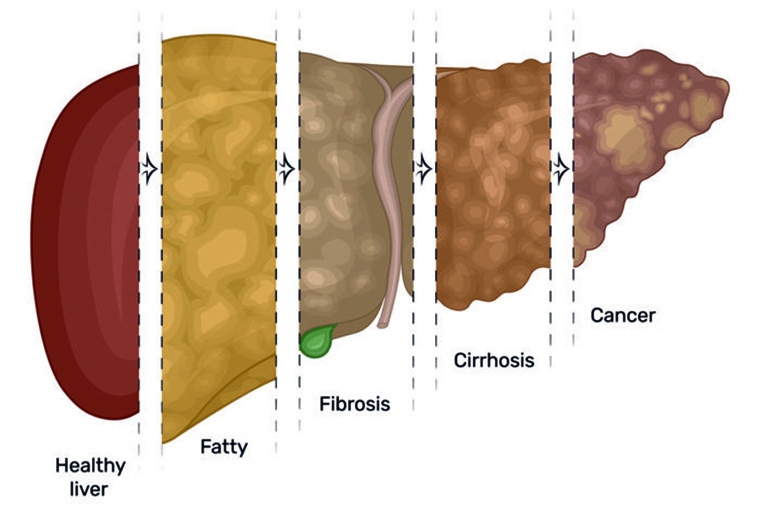
The United States is facing an epidemic of liver disease linked to obesity. Cases of nonalcoholic fatty liver have more than doubled in the past two decades, now affecting around one quarter of the country’s population. The condition leads to inflammation and scarring in the liver, similar to that caused by alcohol abuse, and increases the risk of liver cancer.
Researchers have understood little about how fatty liver causes increased risk of liver cancer, but a new study at Washington University School of Medicine in St. Louis indicates that an RNA binding protein involved in regulating lipid levels in the liver and blood promotes development and progression of fatty liver disease and liver cancer in mice.
The findings, published online in The Journal of Clinical Investigation, shed light on what might lead to such diseases in people, and eventually may contribute to therapeutic interventions for these conditions.
Nonalcoholic fatty liver disease is believed to affect as many as a billion people in the world, some 80 million to 100 million of them in the U.S. Meanwhile, about 40,000 individuals are diagnosed with liver cancer each year in the U.S. — a number that has tripled during the last two decades.
“With the burgeoning epidemic of obesity and nonalcoholic fatty liver disease — both of which are difficult to treat because they require people to make lifestyle changes that allow them to lose weight — it’s vital that we understand these associations,” said senior investigator Nicholas O. Davidson, MD, director of the university’s Division of Gastroenterology. “It’s been difficult to determine how liver cancer develops and why some people with fatty liver disease progress while others don’t because we haven’t had a good way to model nonalcoholic fatty liver disease in the laboratory. These new findings allow us to connect some of the dots linking fatty liver disease to cancer.”
Davidson’s laboratory studies how certain RNA binding proteins — proteins that play a role in various cellular processes such as function and transport — regulate the RNA building blocks that contribute to the synthesis of proteins involved in liver cancer and other gastrointestinal diseases. Davidson studies the genetic regulation of metabolism in the intestine and the liver, with a particular focus on obesity and fatty liver. His recent studies recently have focused on how a particular RNA binding protein called Apobec1 complementation factor (A1CF) operates in the liver.
Previously, his lab genetically engineered mice that don’t make this protein. In the new experiments, the scientists engineered mice that make extra amounts of the protein in hepatocytes, which make up about 80% of the cells in the liver. The mice produced about two to three times the normal amount of the protein.
The researchers found that mice with excessive amounts of the protein developed fatty liver disease even when eating a low-fat diet, and as the mice aged, most developed liver cancer.
Davidson, the John E. and Adaline Simon Professor of Medicine and of Developmental Biology, also directs the Digestive Disease Research Core Center. After noticing that mice with extra amounts of the protein developed fatty liver disease and liver cancer, he collaborated with colleagues at the Mayo Clinic and at the University of Hong Kong, to examine tissue samples from patients who had liver cancer. The researchers found that these tissue samples also contained elevated levels of the protein.
“We found that as these transgenic mice aged, they developed fatty liver spontaneously, regardless of their diet, though the condition was worse in mice that ate a high-fat, high-fructose diet,” he said. “As the animals got even older, the mice developed scarring and fibrosis of the liver, and eventually, most developed liver cancer. When we looked at liver cancer tissue from humans with fatty liver disease, we found extra amounts of the same RNA binding protein there, too.”
Davidson said the findings suggest that in addition to being involved in the regulation of lipids, the protein also plays a role in the development of liver cancer.
“Liver cancer has been difficult to study because of regional differences throughout the liver, and there isn’t a dominant set of tumor drivers such as with other types of cancer, like breast or colon cancer,” he said. “A better understanding of how the overexpression of this RNA binding protein promotes liver cancer could be important to learning how fatty liver disease contributes to cancer risk and perhaps to predict which tumors in the liver might be more aggressive.”
Blanc V, Riordan JD, Soleymanjahl S, Nadeau JH, Nalbantoglu I, Xie Y, Molitor EA, Madison BB, Brunt EM, Mills JC, Rubin DC, Ng IO, Ha Y, Roberts LR, Davidson NO. Apobec1 complementation factor overexpression promotes hepatic steatosis, fibrosis, and hepatocellular cancer. The Journal of Clinical Investigation, Jan. 4, 2021.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute of the National Institutes of Health (NIH). Grant numbers DK112378, DK119437, P30 DK52574, DK106382, DK105129, DK094989 and CA230282. Additional support in a grant from the University of Hong Kong: RGC TRS T12-704/16-R.
Washington University School of Medicine’s 1,500 faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is a leader in medical research, teaching and patient care, ranking among the top 10 medical schools in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.