Condensation might ruin a wood coffee table or fog up glasses when entering a warm building on a winter day, but it’s not all inconveniences; the condensation and evaporation cycle has important applications.

Water can be harvested from “thin air,” or separated from salt in desalination plants by way of condensation. Due to the fact condensing droplets take heat with them when they evaporate, it’s also part of the cooling process in the industrial and high-powered computing arenas. Yet when researchers took a look at the newest method of condensation, they saw something strange: When a special type of surface is covered in a thin layer of oil, condensed water droplets seemed to be randomly flying across the surface at high velocities, merging with larger droplets, in patterns not caused by gravity.
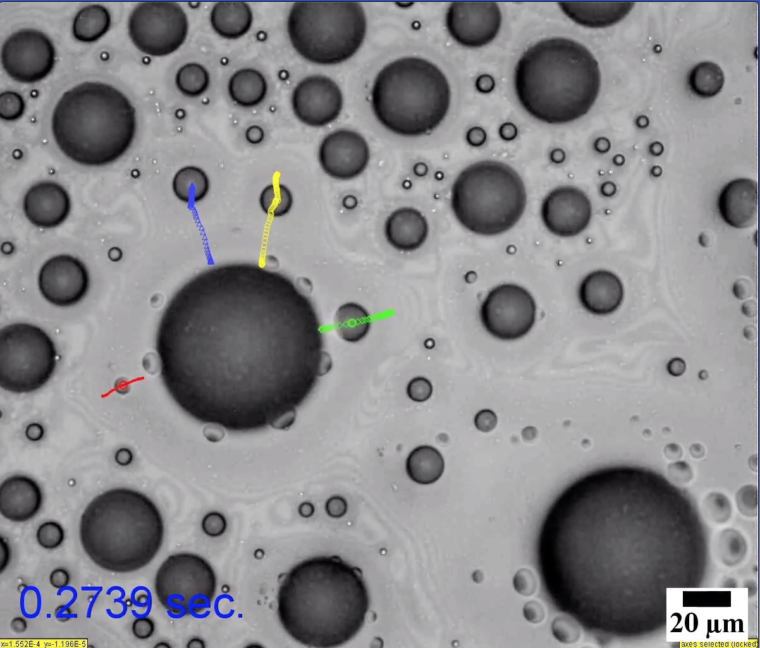
“They’re so far apart, in terms of their own, relative dimensions” — the droplets have a diameter smaller than 50 micrometers — “and yet they’re getting pulled, and moving at really high velocities,” said Patricia Weisensee, assistant professor of mechanical engineering & materials science in the McKelvey School of Engineering at Washington University in St. Louis.
“They’re all moving toward the bigger droplets at speeds of up to 1 mm per second.”

Weisensee and Jianxing Sun, a PhD candidate in her lab, have determined that the seemingly-erratic movement is the result of unbalanced capillary forces acting on the droplets. They also found that the droplets’ speed is a function of the oil’s viscosity and the size of the droplets, which means droplet speed is something that can be controlled.
Their results were published online in Soft Matter.
‘Why are they moving?’
In the most common type of condensation in industry, water vapor condenses to form a thick layer of liquid on a surface. This method is known as “filmwise” condensation. But another method has been shown to be more efficient at promoting condensation and the transfer of heat that comes along with it: dropwise condensation.
It has been used on traditionally hydrophobic surfaces — those that repel water such as the Teflon coating on a non-stick pan. However, these traditional non-wetting surfaces degrade rapidly when exposed to hot vapor. Instead, a few years ago, researchers discovered that infusing a rough or porous hydrophobic surface with a lubricant, such as oil leads to faster condensation. Importantly, these lubricant-infused surfaces (LIS) led to the formation of highly mobile and smaller water droplets, which are responsible for most of the heat transfer when it comes to condensation and evaporation.
During the process, however, the movement of water droplets on the surface seemed erratic — and fast. “They move at a really high velocity for their size,” — about 100 microns —”just by sitting there,” Weisensee said.
“The question is, ‘Why are they moving?’ ”
Using high-speed microscopy and interferometry to watch the process play out, Weisensee and her team were able to discern what was happening and the relationships between droplet size, speed and oil viscosity.
They created water vapor and watched as small droplets formed on the surface. “The first process is that small droplets coalesce and form bigger droplets,” Weisensee said. Capillary forces cause the oil to grow up and over the droplets, forming a meniscus — not the knee muscle, but rather a curved layer of oil surrounding the droplet.
The oil is continuously moving around, trying to strike a balance as it covers different-sized droplets in different places on the surface — if a large droplet forms here, the meniscus stretches over it, causing the oil layer to contract somewhere else. Any smaller droplets in the area of contraction are swiftly pulled to the larger droplets, leading to oil-rich and oil-poor regions.
During the process, larger droplets are essentially clearing the space, which in turn makes room for the formation of more small droplets.

Since most of the heat transfer (about 85 percent) occurs via these small droplets, using LIS for dropwise condensation should be a more efficient way to disperse of heat and get water from vapor. And since the droplets are very small, less than 100 microns in diameter, condensation can occur in a smaller area.
There’s another benefit, too. During “traditional” condensation, gravity is the force that clears water from the surface, making room for new droplets to form. The surface is placed vertically, and the water simply runs off. Since capillary forces are doing the work in dropwise condensation on liquid-infused surfaces, however, the orientation of the surface is of no consequence.
“It could potentially be used on personal devices,” where orientation is constantly changing, she said, “or in space.” And because the entire process is more efficient than traditional condensation, Weisensee said, “This might be a nice way of clearing up space without having to rely on gravity.”
Going forward, Weisensee’s team will measure heat transfer to determine if the smaller droplets during dropwise condensation on LIS are, in fact, more efficient. They also plan to investigate different surfaces in order to maximize droplet movement.