A diet high in fats and sugars is known for its unhealthy effects on the heart. Scientists now have found that a high-fat, high-sugar diet in mouse mothers before and during pregnancy causes problems in the hearts of their offspring, and that such problems are passed down at least three generations, even if the younger generations only eat a standard mouse chow diet.
The study, from Washington University School of Medicine in St. Louis, is published March 22 in the journal AJP-Heart and Circulatory Physiology.
The study also suggests that diet-induced heart changes in offspring are not only transmitted to offspring by their mothers. Obese mothers’ male offspring that mated with healthy females fed a normal diet also passed on the same heart problems. The specific changes to the heart in these offspring were evident in changes to the heart muscle cells’ energy factories, called mitochondria.
“We know that obesity in pregnant mothers raises the risk of future heart problems for her children,” said co-senior author Kelle H. Moley, MD, a professor of obstetrics and gynecology. “But we have shown, at least in mice, that these heart problems don’t stop with a single generation. They are passed down by both the male and female offspring of obese mothers, even when the offspring eat a normal diet. This was a bit of a surprise — problems with heart mitochondria seemed likely to be passed down only through females, through the mitochondrial DNA present in the egg that we inherit only from our mothers.
“Now that we’ve shown that mouse fathers pass this down as well, we have to start studying changes in the DNA of the nucleus in both the egg and the sperm to make sure we understand all the contributing factors,” she said.
Notably, the researchers found multigenerational heart problems, even when the mouse offspring were not obese and ate a normal diet throughout their lives. Though perhaps revealing some effects of a healthy diet, the severity of the heart problems diminished slightly over the generations of mice that ate standard chow diets, the researchers noted.
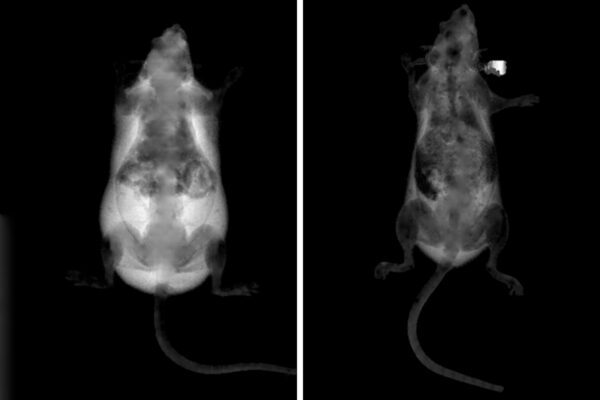
The heart abnormalities induced by maternal obesity included cardiac mitochondria that appeared small and fragmented and that consumed less oxygen than their normal counterparts. The hearts of most of the offspring, though not all, also showed an increase in the weight of the left ventricle, the main pumping chamber of the heart. In people, increased left ventricle weight is often a marker of poor heart muscle quality that predisposes one to heart failure, a potentially fatal condition in which the heart does not pump blood as well as it should.
“The cardiac abnormalities seem to dissipate somewhat over the generations, which is intriguing,” said co-senior author Abhinav Diwan, MD, an associate professor of medicine. “Problems in echocardiograms and the increase in left ventricle mass were less evident in the females of the youngest generation that we studied. There were also differences in male and female hearts that we can’t explain yet. In many ways, this study presents more questions than it answers, and we plan to continue studying these mice to help answer them.”
The researchers also used in-vitro fertilization to implant fertilized eggs from obese mice into normal-weight mice to carry the pregnancies. These offspring also showed the heart defects, demonstrating that the problems are specific to the original egg from the mother fed the high-fat, high-sugar diet, and not the gestational environment during the pregnancy or nursing afterward.
Moley and her colleagues suspect that the defects in heart mitochondria are likely caused by so-called epigenetic changes in the DNA of the original obese mother’s eggs. The epigenome is an important layer of genetic regulation that governs how DNA instructions are read and executed. And, in theory, these epigenetic changes in the egg would be present in every cell of the offspring, including in their male or female reproductive systems. Indeed, past work by this group confirmed that mitochondrial problems also exist in the skeletal muscle, leading to whole-body metabolic abnormalities, such as insulin resistance, in the offspring of obese mouse mothers and in two subsequent generations.
The researchers plan to study the epigenetic changes in the eggs of obese mothers and tissues of the offspring in an effort to understand what is happening to the mitochondria, but in the meantime emphasize the importance of maintaining a healthy weight before and during pregnancy.
“A big question that people may have is, ‘What can I do if my grandmother or great-grandmother was obese?’” said first author Jeremie L.A. Ferey, postdoctoral research scholar. “We need more studies to learn if it’s possible to reverse these mitochondrial defects, but in general, exercise and a healthy diet are always important for heart health.”