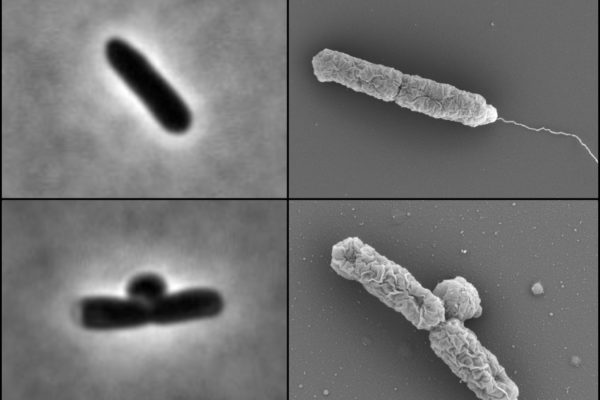
Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics — and at least 23,000 people die as a direct result of these infections.
Petra Levin, professor of biology in Arts & Sciences at Washington University in St. Louis, was recently awarded a $2 million grant to identify and characterize the molecular circuits that coordinate or limit the growth and reproduction of bacteria under changing environmental conditions. This basic research can be used to inform new approaches to defeat antibiotic-resistant infections.

“Cell size control is critical to all domains of life, and size is exquisitely sensitive to changes in environmental conditions, nutritional and otherwise,” Levin said. “Because of this relationship, cell size provides a window into the regulatory circuits that coordinate cell growth with nutrient availability and energy production.”
“Different aspects of growth — including protein synthesis, DNA replication and construction of the bacterial cell wall — are all targets of antibiotics that are used by doctors to fight bacterial infections,” she said.
Levin’s new grant is from the National Institute of General Medical Sciences — the part of the National Institutes of Health that supports basic research to lay the foundation for advances in disease diagnosis, treatment and prevention.
This Maximizing Investigators’ Research Award or MIRA, is designed to provide investigators like Levin with a reliable and stable funding stream for up to five years, allowing them greater flexibility to follow a line of questioning organically. Without such a grant, a researcher might be restricted to the tightly defined set of questions and experiments outlined in a traditional “RO1” proposal.
Size matters
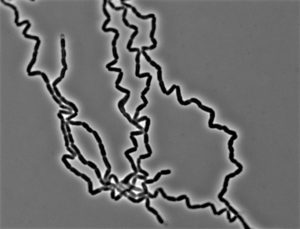
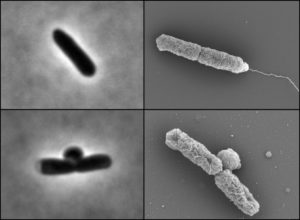
For single-cell organisms, size matters. The availability of nutrients like carbon, nitrogen and phosphate impact how quickly bacteria can synthesize various building blocks and determine how big bacteria can grow before they divide to form daughter cells.
“These cells are very sensitive because they spend lots of time basically all alone, floating out there in the environment,” Levin said. “However the environment changes — that affects them.”
In previous work, Levin’s group demonstrated that the availability of lipids, or fats, determines the maximum size of bacteria and yeast cells. Because lipids are the primary ingredient in the membranes that surround the cells, lipid production restricts cell size. In certain mutants that have lost the ability to coordinate membrane synthesis with synthesis of everything else, treating cells with a drug that inhibits lipid synthesis results in a situation not unlike the Incredible Hulk: the inside of the cell gets bigger and bigger until its “clothing” — the membrane — finally bursts.
Understanding a regulator’s role
A geneticist by training, Levin’s laboratory team features scientists from diverse backgrounds and a toolkit that includes high resolution microscopy, biochemistry, and computational analysis. That enables the research team to perform what she calls a more holistic or “systems level” approach to bacterial physiology, particularly as it relates to cell growth and antibiotic sensitivity.
Since 2013, Levin has served as the co-director of Washington University’s Plant and Microbial Sciences graduate program, and her group is eager to study under the new MIRA grant.
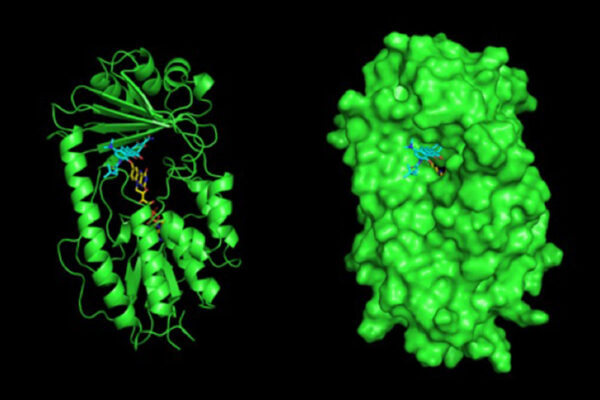
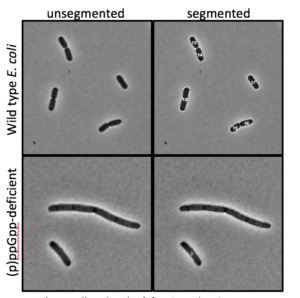
First up, Levin is taking a closer look at the role of a global regulator of cell growth in bacteria: the signalling molecule guanosine tetraphosphate (ppGpp). This molecule helps make sure that synthesis of different cellular components is always in proper proportion, even when cells are under nutritional stress.
Already the Levin research group is working on a few projects related to the commercial antimicrobial triclosan, a substance that used to be ubiquitous in antibacterial soaps, and is still used at high concentration in some toothpastes and certain hospital settings.
Levin and her group recently determined that pre-treatment with triclosan increases the ability of bacteria including E. coli and Staphylococcus to withstand antibiotics up to 10,000-fold, through a ppGpp-dependent mechanism.
Leveraging this finding, Levin will use genetic strategies to identify ppGpp-dependent changes in bacterial physiology that allow cells to survive normally lethal concentrations of antibiotics.
In other new work, Levin and Elizabeth Mueller, a PhD candidate in her lab, have recently discovered why E. coli bacteria encode so many redundant enzymes in their cell wall. Disabling them might be a key to fighting intrinsic resistance to cell-wall-active antibiotics.
“We are developing a clear, integrated picture of homeostatic regulatory circuits that coordinate nutrient availability, cell growth and the cell cycle in bacteria,” Levin said.