A twisted ankle, broken hip or torn knee cartilage are all common injuries that can have medical ramifications long after the initial incident that causes them. Associated pain, inflammation, joint degeneration and even osteoarthritis can sideline a variety of different people: athletes, weekend warriors and patients who are either aging or inactive.
A team from Washington University in St. Louis was awarded $1.7 million from the National Institutes of Health (NIH) to develop a new therapeutic treatment that can deliver disease-modifying compounds in a manner to delay the development of inflammation, joint degeneration and arthritis with all the associated discomfort, disability and pain.

“We’re starting to see that many areas can’t be reached via oral drug delivery,” said Lori Setton, the Lucy & Stanley Lopata Distinguished Professor of Biomedical Engineering at the School of Engineering & Applied Science. “For example, synovial joint fluid in the knee is almost optimized to rapidly clear compounds out of the joint. So we’re trying to trick the joint into being a good host for the therapeutic drugs we are delivering.”
Setton, whose lab focuses on the role of mechanical factors in the breakdown and repair of soft tissues, said an intracellular compound called nuclear factor kappa B (NF-kB) is a main culprit in cellular breakdown, inflammation and pain after an injury. She’s working in the lab on a new solution using silk to deliver two specific molecules that can inhibit NF-kB at the site of a fracture or injury in an effort to stave off long-term joint damage.
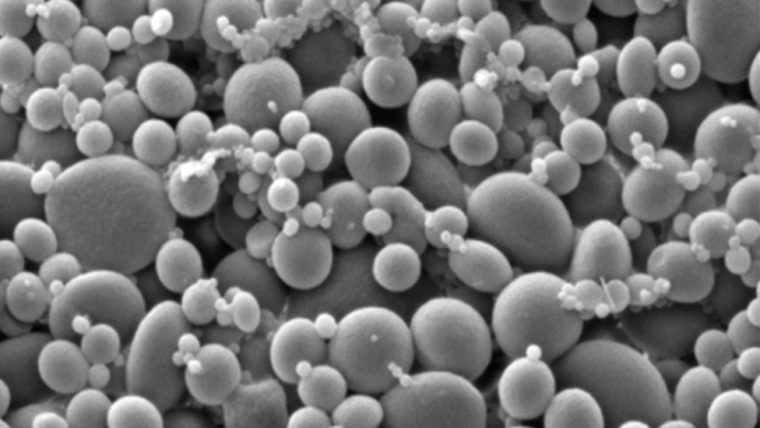
“Silk naturally doesn’t interact with water, and, when you mix it with these molecules that also don’t interact with water, they bind to each other very strongly,” Setton said. “We believe these selective compounds are therapeutically effective, but we’ve never been able to get them to their target site. By delivering them with the silk, we hope to get large doses to the target site with low toxicity and to have them remain in that compartment for longer periods of time.”
In preliminary work with Tufts University investigator David Kaplan, Setton showed that model compounds can reside in the joint space about five times longer if delivered with silk microparticles than if delivered alone. Silk is an attractive delivery vehicle because of its long history of safe clinical use, and Kaplan has received NIH support to promote translational uses of silk for medical and other applications. It was initial work in delivering silk to the knee joint that drove Setton to identify a suitable, disease-modifying compound for treatment of arthritis through collaborations with the Musculoskeletal Research Center at the Washington University School of Medicine.
Setton and her co-investigators at the School of Medicine — including Yousef Abu-Amer, professor of orthopaedic surgery; Farshid Guilak, professor of orthopaedic surgery; and Gabriel Mbalaviele, associate professor of medicine in the Division of Bone and Mineral Diseases — soon will start testing the new delivery system in animal models.
“Delivering drugs orally to combat NF-kB-mediated problems at specific locations in the body, such as the injured knee, can be associated with harmful biological functions,” Abu-Amer said. “So this type of site-targeted approach to inhibit elevated NF-kB is essential if we want to provide effective treatment to the targeted site.”
According to Setton, the enhanced drug-delivery system has the potential to prevent the onset and progression of joint damage in patients suffering from acute injuries, like minor joint fractures, ligament or meniscal tears.
“Patients with joint trauma tend to go on to develop osteoarthritis at a higher rate compared to someone who doesn’t have the injury,” Setton said. “It’s a whole different type of arthritis development that we don’t know a whole lot about, but we believe we can intervene early with new drug delivery and treatments, and prevent onset at a later stage.”