Nanoparticles carrying a toxin found in bee venom can destroy human immunodeficiency virus (HIV) while leaving surrounding cells unharmed, researchers at Washington University School of Medicine in St. Louis have shown. The finding is an important step toward developing a vaginal gel that may prevent the spread of HIV, the virus that causes AIDS.
“Our hope is that in places where HIV is running rampant, people could use this gel as a preventive measure to stop the initial infection,” says Joshua L. Hood, MD, PhD, a research instructor in medicine.
The study appears in the current issue of Antiviral Therapy.
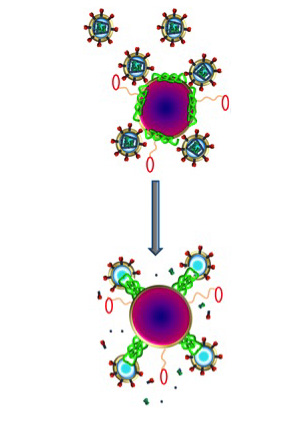
Bee venom contains a potent toxin called melittin that can poke holes in the protective envelope that surrounds HIV, and other viruses. Large amounts of free melittin can cause a lot of damage. Indeed, in addition to anti-viral therapy, the paper’s senior author, Samuel A. Wickline, MD, the J. Russell Hornsby Professor of Biomedical Sciences, has shown melittin-loaded nanoparticles to be effective in killing tumor cells.
The new study shows that melittin loaded onto these nanoparticles does not harm normal cells. That’s because Hood added protective bumpers to the nanoparticle surface. When the nanoparticles come into contact with normal cells, which are much larger in size, the particles simply bounce off. HIV, on the other hand, is even smaller than the nanoparticle, so HIV fits between the bumpers and makes contact with the surface of the nanoparticle, where the bee toxin awaits.
“Melittin on the nanoparticles fuses with the viral envelope,” Hood says. “The melittin forms little pore-like attack complexes and ruptures the envelope, stripping it off the virus.”
According to Hood, an advantage of this approach is that the nanoparticle attacks an essential part of the virus’ structure. In contrast, most anti-HIV drugs inhibit the virus’s ability to replicate. But this anti-replication strategy does nothing to stop initial infection, and some strains of the virus have found ways around these drugs and reproduce anyway.
“We are attacking an inherent physical property of HIV,” Hood says. “Theoretically, there isn’t any way for the virus to adapt to that. The virus has to have a protective coat, a double-layered membrane that covers the virus.”
Beyond prevention in the form of a vaginal gel, Hood also sees potential for using nanoparticles with melittin as therapy for existing HIV infections, especially those that are drug-resistant. The nanoparticles could be injected intravenously and, in theory, would be able to clear HIV from the blood stream.
“The basic particle that we are using in these experiments was developed many years ago as an artificial blood product,” Hood says. “It didn’t work very well for delivering oxygen, but it circulates safely in the body and gives us a nice platform that we can adapt to fight different kinds of infections.”
Since melittin attacks double-layered membranes indiscriminately, this concept is not limited to HIV. Many viruses, including hepatitis B and C, rely on the same kind of protective envelope and would be vulnerable to melittin-loaded nanoparticles.
While this particular paper does not address contraception, Hood says the gel easily could be adapted to target sperm as well as HIV. But in some cases people may only want the HIV protection.
“We also are looking at this for couples where only one of the partners has HIV, and they want to have a baby,” Hood says. “These particles by themselves are actually very safe for sperm, for the same reason they are safe for vaginal cells.”
While this work was done in cells in a laboratory environment, Hood and his colleagues say the nanoparticles are easy to manufacture in large enough quantities to supply them for future clinical trials.
Editor’s note: No bees were harmed in this study because the researchers used a synthetic form of the bee venom toxin.
Hood JL, Jallouck AP, Campbell N, Ratner L, Wickline SA. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antiviral Therapy. Vol. 19: 95 – 103. 2013
This work was supported by the Bill & Melinda Gates Foundation Grand Challenges Explorations grant number OPP1024642 ‘Fusogenic nanoparticles for combined anti-HIV/contraception.’
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.