A 78-year-old St. Louis woman was the first patient in this region to receive an experimental device to replace her defective aortic valve without opening the chest wall or using a heart-lung machine. This procedure was performed by Washington University heart specialists at Barnes-Jewish Hospital Jan. 15.
The woman is an initial participant in a national multicenter trial to evaluate the effectiveness of the new device. If proven effective, this new device holds enormous hope for patients who are unable to undergo the standard open-heart surgery for aortic valve replacement because they are too old or too sick to qualify for the surgery.
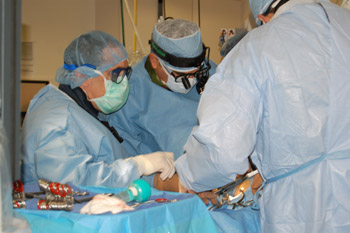
The School of Medicine and Barnes-Jewish Hospital are one of only 10 heart centers selected by the U.S. Food and Drug Administration to evaluate this technique, which uses a catheter to thread a replacement aortic valve into the heart. Mounted on a catheter, the valve can be guided through the patient’s circulatory system from the leg or inserted between the ribs into the heart and expanded at the site of the patient’s diseased valve. The technique is called transcatheter valve replacement.
“Pending the study’s outcome, this has the potential to be one of the most significant advances in all of cardiac medicine,” said John M. Lasala, M.D., Ph.D., principal investigator of the trial, professor of medicine and medical director of the Cardiac Catheterization Laboratory at Barnes-Jewish Hospital.
If the device lives up to that potential, it could benefit many of the roughly 200,000 patients per year in the United States who need a new heart valve because they have severe aortic stenosis, a narrowing of the aortic valve, which normally ensures the efficient flow of blood out of the heart and into the body. Severe aortic stenosis can lead to congestive heart failure and sudden death.
The clinical trial, called the PARTNER trial (Placement of AoRTic traNscathetER valves), will eventually enroll about 600 patients at up to 15 sites nationwide. The device, developed by Edwards Lifesciences, consists of a heart valve made of cow heart tissue attached to a collapsible mesh cylinder. “Partner” also signifies the partnership between a cardiac surgeon and an interventional cardiologist, both of whom participate in each patient’s procedure. Interventional cardiology is a specialty that deals with the catheter-based treatment of structural heart diseases.
In addition to Lasala, physicians conducting the trial at the School of Medicine are Ralph J. Damiano Jr., M.D., the John M. Shoenberg Professor of Surgery, chief of cardiac surgery and a cardiac surgeon at Barnes-Jewish Hospital; Nader Moazami, M.D., associate professor of surgery and chief of cardiac transplantation; and Alan Zajarias, M.D., assistant professor of medicine in the cardiovascular division.
“Open-heart surgery for aortic valve replacement is a very common procedure, but many of those who need it are too old or too sick to qualify for the surgery,” Damiano said. “That fact led to the development of these transcatheter valves. An earlier, small feasibility study showed that the mortality rate with the valves was nearly as low as that of conventional valve replacement surgery — around 10 percent.”
The transcatheter technique does not require stopping the heart and placing the patient on a heart-lung bypass machine, so frail or sick patients can tolerate the procedure. Without valve replacement, the life expectancy of patients with severe aortic stenosis is very short, usually less than five years.
Two groups of patients will take part in the PARTNER trial. The surgical arm of the trial will consist of patients with severe aortic stenosis who are considered at high-risk because of their disease, but who nevertheless are candidates for conventional open-heart surgery. The other arm will consist of patients with severe aortic stenosis who are considered inoperable because they are unlikely to survive open-heart surgery.
In the surgical arm, patients will be randomly assigned to receive either the transcatheter valve or to undergo a conventional valve replacement in which the chest cavity is opened and a new valve is sewn into the heart. In the other arm of the trial, patients will be randomly assigned to receive either the transcatheter valve or appropriate medical therapy.
Success in the surgical arm is achieved if the clinical results demonstrate that the transcatheter heart valve is not statistically inferior to conventional open-heart surgery. The clinical results of the other arm need to demonstrate that the transcatheter heart valve is statistically superior to medical management.