Physicians say that smoking is by far the biggest cause of emphysema, but why doesn’t every smoker get the disease? If you asked Michael Holtzman, M.D., that question, he might answer that for most cases of emphysema you need a mix of genes, viruses and cigarettes.
w5-jwjZ3I2Y
Emphysema and the associated condition of chronic bronchitis are both disorders that contribute to chronic obstructive pulmonary disease (COPD), which is the fourth leading cause of death in the United States. Research by Holtzman and his colleagues at Washington University School of Medicine in St. Louis suggests that someone destined to suffer from COPD may start with a susceptible genetic makeup and then experience a severe viral lung infection in early childhood. The infection could “reprogram” the cells of the lung’s air passages and sacs, and the reprogrammed cells could react badly if the same person took up cigarette smoking, leading to COPD some time down the road.
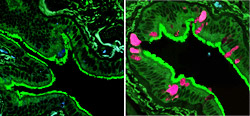
On the left is an image of microscopic airways in normal lung tissue. On the right is lung tissue from a patient with COPD. The pink color highlights cells producing excess mucus, a symptom of COPD.
“Cigarette smoking has created a very large population of COPD patients worldwide,” he says. “At present, we can treat them with steroids to reduce inflammation, antibiotics to suppress infections, and oxygen to help their breathlessness, but the disease will still progress until it’s fatal. We need to find treatments that stop the disease progression and to do that we need a much better understanding of how COPD develops.”
Now Holtzman and his colleagues at the School of Medicine have obtained funds from National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) totaling $14.9 million to establish a Specialized Center for Clinically Oriented Research (SCCOR), an ambitious type of grant program meant to foster research that can quickly apply basic science findings to clinical problems.
Holtzman’s SCCOR program will take a comprehensive look at the molecular changes that occur as lungs become crippled by COPD — a disease that affects at least 16 million people in the United States today.
Lungs have a tree-like structure of intricately branching airways ending in tiny sacs or alveoli, which exchange gases between the blood and the air. In chronic bronchitis, airways overproduce mucus and become inflamed, obstructing airflow. In emphysema, alveoli are destroyed so they can no longer take up oxygen from the air. COPD patients can have both problems at once, and Holtzman and his colleagues are studying both issues.
| COPD Facts |
For more information go to learnaboutcopd.org |
“A key project, led by Richard Pierce (Ph.D., research associate professor of medicine) examines the structural defects found in COPD and correlates them to changes at the molecular level,” says Holtzman, who is the Selma and Herman Seldin Professor of Medicine and director of the Division of Pulmonary and Critical Care Medicine.
Researchers will use newly developed imaging techniques, such as helium MRI, to look at the tissue of lungs removed from COPD patients undergoing lung transplants and home in on the tiny lung structures that are injured. “Then, if we find that a particular gene is overactive at a site where the disease is particularly severe, we’ll analyze the normal and abnormal function of that specific gene,” Holtzman says.
Holtzman notes that so far no other researchers have done this because it takes a combination of new imaging techniques, advanced gene analysis technologies and a highly active lung transplant program — all of which are on hand at the School of Medicine.
A defining characteristic of COPD is the breakdown of the fibrous protein elastin, whose stretchiness gives the lung its elastic properties. Damage to elastin prevents air sacs from deflating properly.
Holtzman’s colleague Zsolt Urban, Ph.D., assistant professor of pediatrics and genetics, has identified a mutant gene responsible for abnormal elastin in humans and has teamed with Robert Mecham, Ph.D., who has engineered mice that carry the mutant elastin gene. These mice and others with different variants of the elastin gene will enable the researchers to probe the malfunctions associated with abnormal elastin genetics in the lungs. Mecham is the Alumni Endowed Professor of Cell Biology and Physiology and professor of internal medicine, pediatrics and biomedical engineering.
The fibrous protein collagen supports the lung’s structure, and both elastin and collagen can be affected if protein-digesting enzymes in the lungs aren’t tightly controlled by their normal inhibitors. Robert M. Senior, M.D., professor of internal medicine and of cell biology and physiology, and colleagues are studying the imbalance that occurs in emphysema between the protein-digesting enzymes (proteases) and their inhibitors (antiproteases).
“In particular, they are targeting a specific protease that hasn’t been well studied but now appears to be the predominant one responsible for the destruction of collagen,” Holtzman explains. “They will examine the factors that determine when and how the protease is produced and activated and how these factors interact with cigarette smoke to induce inflammation of lung tissue and destruction of alveoli.”
A fourth project area, led by Holtzman, addresses why COPD patients overproduce mucus in their airways. Earlier work suggested that a viral infection triggers a population boom in the mucus-producing cells of the airway.
“We have a genetic type of mice that develops lifelong lung disease after a transient viral infection, and the disease that they develop resembles human COPD,” Holtzman says. “The mice especially develop mucous cell metaplasia, an overabundance of mucus-producing cells. We will be carefully defining the steps that lead to this disease trait and then determining if the same process occurs in human patients. This is critical because we have no drugs to treat overproduction of mucus, and this is a frequent cause of the morbidity and mortality in patients with respiratory diseases of all kinds, especially COPD.”
Holtzman says that all the projects under the grant include both experimental models and patient studies. “That’s a mandate for successful SCCOR programs,” Holtzman says. “The basic disease mechanisms that we find in the lab identify the critical diagnostic and therapeutic targets that we can then study in patients. In that way, each project aims to improve both diagnosis and therapy of COPD.”
The NHLBI launched a national awareness and education campaign on COPD in January of this year. More information about the causes and treatments of the COPD can be found at www.learnaboutcopd.org.
Washington University School of Medicine’s full-time and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.