Pain and addiction
Breaking the grip
Pull on any loop of the Gordian knot that is finding treatments for pain and addiction, and the whole knot just tightens. Powerful painkillers come with a daunting risk of chemical dependency. The persistent sadness, worry and inability to find joy in life that can accompany chronic pain make substance use more appealing.
“Understanding pain and addiction is one of the most pressing needs in health care,” says Michael S. Avidan, MBBCh, the Dr. Seymour and Rose T. Brown Professor of Anesthesiology and head of the Department of Anesthesiology. “The way we treat pain has exacerbated the opioid epidemic, which is one of the biggest scourges in our society. We’re not going to solve this by waging a war on drugs. We’re going to address this by understanding the mechanisms of pain, why addiction occurs and by translating scientific insights into clinical practice.”
Pain and pleasure
It’s hard to be happy when you’re always hurting. That’s one reason opioids can be so addictive — they dampen both physical and emotional pain, leaving people temporarily pain-free and euphoric. Neuroscientists Meaghan C. Creed, PhD, and Jose A. Moron-Concepcion, PhD, the Henry Elliot Mallinckrodt Professor of Anesthesiology, have identified circuitry in the brain that links pain to negative emotions such as sadness, anxiety and an inability to feel joy. The finding suggests that modulating the circuitry could treat both emotional and physical suffering, an approach that could reduce the addictive potential of opioids and improve quality of life for people with chronic pain, even when it’s not possible to completely eliminate the pain itself.
“By targeting the emotional aspects of pain, we hope to make pain less debilitating so that patients won’t crave the emotional high they get from opioids,” says Moron-Concepcion.
Sleep quality
Keeping the brain sharp
There’s nothing like a good night’s sleep. Apart from the pleasure of waking up refreshed and relaxed, sleep consolidates memories and enhances learning and creativity, which is why people grappling with a thorny problem are well advised to “sleep on it.” Sleep allows time for the brain’s housekeeping cells to clear away the molecular debris from the day, so the brain can start the next day fresh. And poor sleep doesn’t just make people grouchy; it has been linked to diabetes, heart disease, depression, Alzheimer’s and many other chronic conditions. WashU Medicine scientists are leaders in exploring sleep, from the fundamental question of why we do it in the first place, to how and why sleep disturbances undermine health and what can be done to address it.
Sleep is the best medicine
To Brendan P. Lucey, MD, director of the Washington University Sleep Medicine Center, the goal is to help people sleep better. After his lab and others showed that poor sleep increases brain levels of two damaging Alzheimer’s proteins, he set out to determine whether good sleep could lower the levels of toxic proteins and thereby prevent or delay the onset of cognitive symptoms. He leads an ongoing phase 2 clinical trial of suvorexant, an FDA-approved insomnia drug. Preliminary results have been promising, hinting at the potential of sleep medications to slow or stop the progression of Alzheimer’s disease.
Brain tumors
Fighting with targeted tools
Whether malignant or benign, a brain tumor is life-altering. Malignant tumors can spread and become deadly. And “benign” doesn’t mean harmless; benign tumors can cause serious problems such as paralysis, seizures and personality changes, depending on which parts of the brain they affect.
“Brain tumors affect the fundamental attributes of people — their personality and behavior; their ability to move, speak, see, think — that are just such essential characteristics of a human being,” says neurosurgeon Albert H. Kim, MD, PhD, the founding director of the Brain Tumor Center at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine. “To understand and treat brain tumors, we have to attack them from different angles and tailor therapy to each person.”
Zika’s silver lining
Zika virus made headlines worldwide in 2015, when it caused thousands of babies in Brazil to be born with tiny, misshapen brains. Neuro-oncologist Milan G. Chheda, MD, and virologist Michael S. Diamond, MD, PhD, aim to harness the virus’s infamous power to kill brain cells and direct it against brain cancers. They have shown that the virus can activate immune cells to destroy an aggressive brain cancer in mice, giving a powerful boost to an immunotherapy drug and sparking long-lasting immunological memory that can ward off tumor recurrence for at least 18 months.
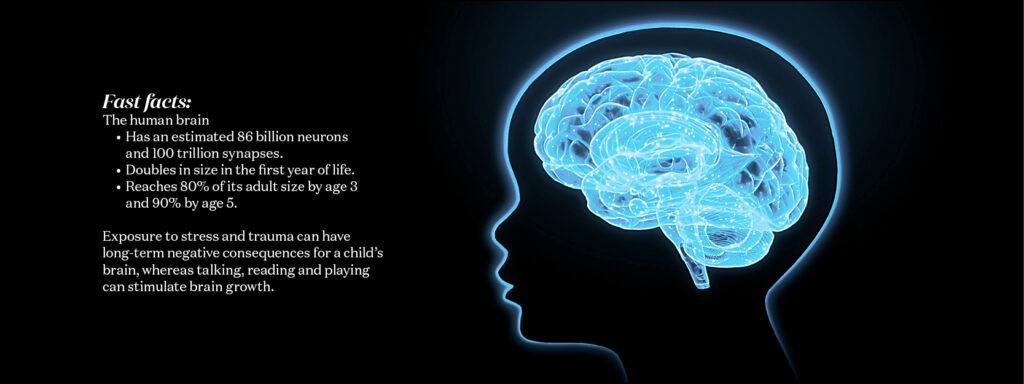
Human development
Starting out strong
The first 1,000 days, from conception to around the second birthday, are a period of exponential brain growth and development. Anything that affects the process — including genetic alterations, chemicals such as alcohol or the home environment — can have lifelong consequences. WashU Medicine researchers are leading efforts to understand the factors that influence brain development to promote healthy brains and help people live their best lives.
Building the brain
How do billions of neurons organize themselves into structures and networks to form the enormously complex machine that is the brain? The answer could shed light on the roots of conditions such as epilepsy, autism and intellectual disability, but studying the developing brain in utero has proved challenging.
Linda J. Richards, PhD, the Edison Professor of Neuroscience and head of the Department of Neuroscience, has pioneered the study of brain development in marsupials that are born 16 days after conception. They are born with brains little more than nubs and undergo the bulk of development after birth. Recently, Richards showed that distinct activity patterns emerge in different brain areas just weeks after conception. “Would alterations to patterned activity disrupt how brain circuits are set up, and if so, could that cause developmental disorders?” she asks.
Neurological injury
Smoothing the road to recovery
A car accident, a gunshot, a stroke — in an instant, everything changes. Returning to the way things were tends to be slow and difficult, with no guarantees. WashU Medicine researchers are making crucial discoveries regarding the biology of regeneration and recovery. These discoveries pave the way toward — and in some cases already have achieved — innovative new therapies and devices to help people recover from neurological injury as fully as possible.
A breakthrough device
The first FDA-approved device that helps stroke patients retrain their brains is based on research by Eric C. Leuthardt, MD, the Shi Hui Huang Professor of Neurological Surgery, and biomedical engineer Daniel W. Moran, PhD. Leuthardt’s insights into motor signaling in the brain led to the development of the IpsiHand, in which patients use a brain-computer interface to control a robotic glove that opens and closes their hands. Repeated use gradually teaches patients’ brains to do it on their own, restoring significant hand function. The FDA designated the IpsiHand a “Breakthrough Device,” which means it meets a critical unmet need, and gave it “de novo” authorization, as there was no similar medical device on the market.
Immune-brain nexus
Wielding a double-edged sword
The brain was once thought to be off-limits to the immune system, too sensitive to tolerate immune cells with their sharp weapons. Now, scientists know that immune cells and molecules flow into and out of the central nervous system all the time, supporting normal brain function, fighting infections and tumors, and, yes, sometimes causing injury and disease. The nexus between the immune and nervous systems provides new opportunities to intervene to promote health and prevent or treat neuroinfectious, neuroimmune and neurological conditions ranging from COVID-19 to multiple sclerosis to autism.
Immune surveillance
The idea that the immune system surveils and protects the brain is now widely accepted, but until recently it wasn’t clear how and where. In 2015, neuroscientist and immunologist Jonathan “Jony” Kipnis, PhD, found a network of vessels in the meninges — the tissues encasing the brain — that drains fluid and small molecules from the brain into the lymph nodes. Subsequent research indicated that immune cells stationed in the meninges inspect fluid as it washes out of the brain. Such cells are prepared to initiate an immune response if they detect signs of infection or injury, Kipnis says. “The immune cells that sit on the borders of the brain could potentially be a feasible target for treating neurological diseases such as Alzheimer’s, once we better understand their role in these complex diseases.”
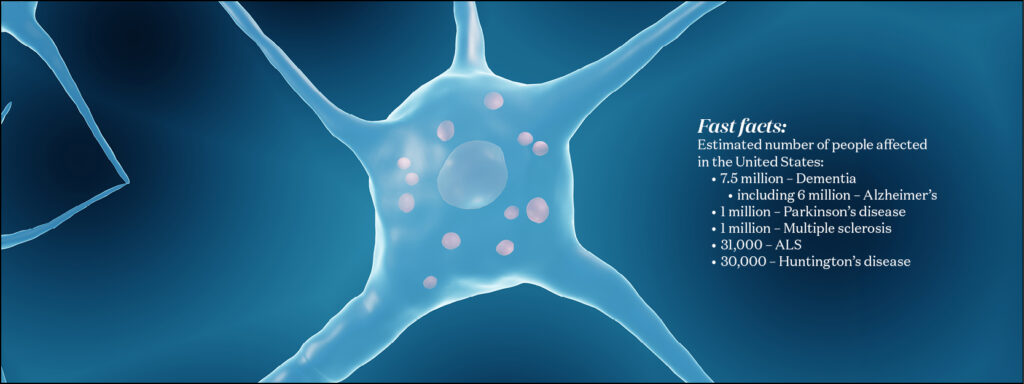
Neurodegeneration
Preserving function
Neurodegeneration tends to start slowly, almost imperceptibly. For a variety of reasons, neurons gradually stop functioning as well as they used to, and some start dying. In Alzheimer’s disease, the most common neurodegenerative disease, signs of decay are detectable long before memory issues arise. This slow start provides an opportunity to intervene while the problem is still manageable. WashU Medicine researchers are working on detecting and healing ailing neurons, opening paths to new treatments.
Therapy for few; hope for many
In April 2023, the FDA approved the drug tofersen — based on international clinical trials led by Timothy M. Miller, MD, PhD, the David Clayson Professor of Neurology — for an inherited form of amyotrophic lateral sclerosis (ALS), a paralyzing neurological disease. The drug targets a gene called SOD1, and clinical trials show that it slows disease progression in the 2% of ALS patients with SOD1 mutations. The drug’s success provides new hope for disease-changing therapies for other forms of ALS, once thought to be untreatable. “My whole career has been built on the assumption that ALS is a treatable disorder,” Miller says.