Often, war metaphors are forced. This one is not. We are fighting invisible enemies, one all too obvious, the others a guerrilla force that can show up anywhere, at any time. SARS-CoV-2 landed fast and caught us off guard. But with drug-resistant infections, we armed the enemy ourselves — and the casualties are quietly mounting.
Because of the way we have overused antimicrobial drugs (antibiotics, antivirals, antifungals), they are now useless against more and more infections. And because we no longer have effective treatments, these infections are killing 35,000 people a year in the U.S. alone, says the latest and most alarming report from the Centers for Disease Control and Prevention. The United Nations estimates that by 2050, the global death toll could reach 10 million — almost double the number of lives lost thus far to COVID-19.
The acronym for six of the most dreaded pathogens is ESKAPE. The acronym for a new bill to encourage drug research and development is DISARM. And the warfare feels like terrorism, relocating and shapeshifting by the second. When a new coronavirus emerged in China and swiftly spread to other countries, we had no antiviral drugs ready to treat it, and we had to move fast to develop vaccines. Since then, SARS-CoV-2 has mutated, changing as much, on a viral scale, as our species has changed in the past 2.5 million years.
We have managed to keep up, barely. But with many other infections to deal with, we remain outgunned, our health-care troops waiting in desperation for new drugs and diagnostic tests.
The casualties of resistant infections take many forms. A 4-year-old girl comes close to dying from a trivial scratch. A preemie develops a blood infection, and the bacteria grow more resistant with each treatment. Five teenagers taking the combo antibiotic Bactrim (sulfamethoxazole plus trimethoprim) develop a respiratory illness so severe they need a heart-lung machine to stay alive. A patient in his early 20s, partially paralyzed, develops a spine infection that resists everything except colistin — a drug physicians had stopped using in humans long ago because it damages the kidneys.
Researchers at Washington University School of Medicine — along with researchers in molecular microbiology and synthetic biology on the Danforth Campus — were already mobilized against these threats when the pandemic hit. One lab, for example, had identified a new set of enzymes that would inactivate the oft-used antibiotic tetracycline. They had also found a way to counteract the enzymes’ effect. Others were working on vaccines against staph and E. coli; reversing drug-resistant TB; using viruses to attack bacteria; performing fecal microbiome transplants (using the good bacteria from a healthy person’s waste to attack a C. diff. infection); and synthesizing molecules that can mimic the immune system.
Then, in 2020, research at the university and around the world pivoted to SARS-CoV-2. It was tough to stop some important non-COVID projects midstream, but no one doubted the urgency. And the speed and cooperation, the strong public-private partnerships and the public health outreach showed us what we were capable of.
Now we need a collaboration of the same magnitude to attack antimicrobial resistance.
Antibiotics were once miracle drugs. They made it possible for wounded soldiers not only to survive but to avoid losing a limb to gangrene; lengthened the lives of patients with cystic fibrosis; allowed surgeons to safely do heart and lung transplants; helped people recover from once-deadly pneumonia. Today, we have a far longer list of miracles: We can stop cancer with targeted treatments and immunotherapies, turn HIV from a death sentence into a manageable chronic illness, ease the torment of autoimmune diseases with new biologic drugs, replace shattered joints and do complex surgeries. But because we have overused antibiotics, treating them as “magic bullets,” many of these advances are in jeopardy. Medicine can do all that is necessary to save a life, only to lose it to a drug-resistant infection.
“The public health threat of antibiotic resistance is not some mystical apocalypse or fear-mongering. The epidemic of antibiotic resistance is a current reality. It’s affecting patients right here, right now. Many physicians are faced with trying to take care of patients who have drug-resistant infections that we have no treatment for.”
— Victoria Fraser, MD, the Adolphus Busch Professor of Medicine and chair of internal medicine, told NBC two years ago.
The COVID pandemic made antibiotic resistance an even greater danger, Fraser says now. Nationwide, “broad-spectrum antibiotic use increased during COVID surges, and infection-prevention resources were diverted from antimicrobial stewardship to COVID precautions. There was also a tremendous shortage of personal protective equipment.”
Why did it take so long, even before COVID, to galvanize a response to the global epidemic of antibiotic resistance? “Because for many people, antibiotics are still remarkably effective,” says William Powderly, MD, co-director of the Division of Infectious Diseases at the School of Medicine and the Larry J. Shapiro Director of the Institute for Public Health. After suffering a miserable sinus infection for a few days, you cave and call the doctor, begin a course of antibiotics, and start to feel better. Maybe you would have anyway, but you credit the drug and ask for it by name the next time. A toddler gets an ear infection — who wants to see her suffer? A woman gets a urinary tract infection and is prescribed a week of antibiotics to be on the safe side. Yet “for healthy women who develop a UTI, often a single dose of an antibiotic is enough, and certainly no longer than three days,” adds Powderly, also the Dr. J. William Campbell Professor of Medicine.
In this country — so far — resistance usually only threatens patients with more serious infections or extra vulnerability: those who are very young or very old, or who live with obesity, diabetes or compromised immune systems. But serious infections are increasing, and the more antimicrobials we use, the more resistance is created worldwide.
“We are now seeing something we didn’t see five years ago: infections for which we have no antibiotics or maybe only a partially effective one,” Powderly says, his voice grim. “And in most cases, these infections are fatal.”
Knowing the enemy
Antimicrobial resistance is far older than the medicines we blame. A resistant virus was buried for 30,000 years in the Siberian permafrost; resistant bacteria hid in one of the world’s deepest caves for more than 4 million years. They already possessed natural properties of resistance — and those properties can spread. What happens when we overuse antimicrobials, though, is that we kill off all the nonresistant organisms, leaving a clear field for those with resistance to grow and replicate. We help natural selection strengthen our enemy.
“Even health-care professionals don’t always understand,” says Caline Mattar, MD, assistant professor of medicine, who helped develop a curriculum for health professionals for the World Health Organization. “Some still thought it was the body that becomes resistant, not the bacteria. Others thought resistance was not a problem in their country or practice. Antibiotic resistance is a problem everywhere.”
The pandemic set back a lot of progress, Mattar adds. “The laboratory surveillance structure was repurposed, the supply chain — even for old antibiotics — collapsed, stewardship programs were shut down or repurposed. It was just not a priority anymore.” Even if “normalcy” returns, the current model of antibiotic development and reimbursement (more on that later) means that “the population who will need new antibiotics the most — in low- and middle-income countries — is unlikely to be able to access them.”
Resistance, meanwhile, increases worldwide, driven by the profligate use of antibiotics in agriculture. Mattar winces at the quantity of antimicrobials used to stimulate the growth of Florida’s citrus trees and the copious antibiotics used to promote growth and prevent infection in livestock (approximately 80% of all antibiotic use in the U.S.). In 2019, the CDC counted 765 oral outpatient antibiotic prescriptions per thousand people in the U.S.
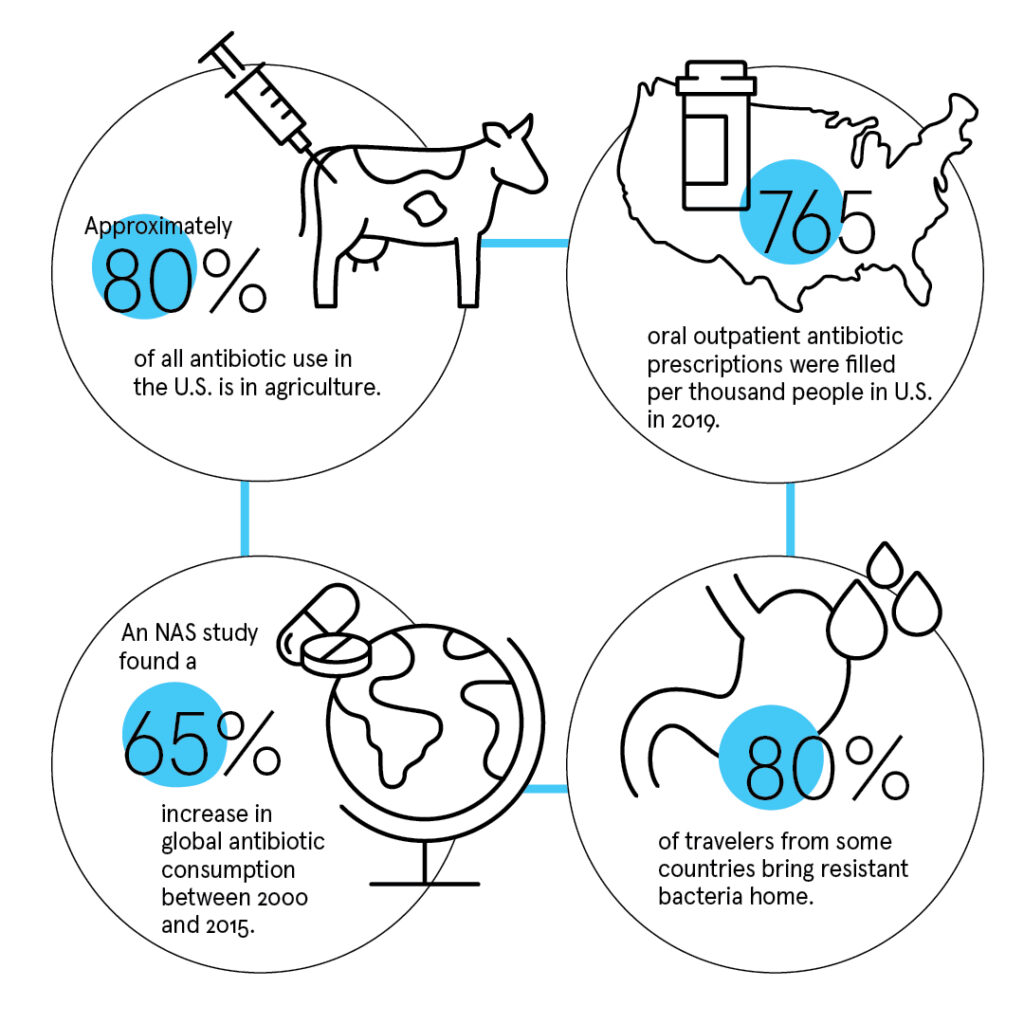
Some countries are so rife with resistant bacteria that 80% of travelers carry them home unwittingly, colonized in their guts after a gulp of water or a few bites of raw banana.
In India, where Sumanth Gandra, MD, associate professor of medicine, has been researching resistance since 2012, antibiotics are available over the counter at drugstores, and they are being heavily prescribed during the pandemic — despite the fact that SARS-CoV-2 is a virus, and antibiotics attack bacteria. They are also used in vast quantities to raise poultry, and both antibiotics and resistant bacteria enter groundwater and can contaminate the water supply. In many countries with limited resources, environmental sanitation is poor, so bacteria spread easily. Pollution inflames the lungs, making people more vulnerable to respiratory infections. Cultural practices and behavior can also increase transmission of resistant bacteria; for example, millions of people take ritual baths in the River Ganges for religious reasons.
“If a disease is not a problem in western Europe or the U.S., there is no incentive for pharmaceutical companies to discover new drugs,” Gandra says flatly. Meanwhile, “in low-income countries, the use of antibiotics is increasing.” He co-authored a study, published by the National Academy of Sciences, that found a 65% increase in global antibiotic consumption between 2000 and 2015, driven by rising rates of antibiotic use in low-income countries.
“A child in one of these countries will get 25 courses of antibiotics prescribed in the first five years of life, and most of the time, it’s not necessary.”
— Sumanth Gandra, MD, associate professor of medicine
As for preventing the spread of infection in hospitals, “there are no single rooms, and more people have resistant bacteria in their gut than don’t,” Gandra says. “Addressing this problem would require reverse isolation — to try to protect the person who doesn’t have resistant bacteria.”
In her lab at the med school, Jennie H. Kwon, DO, assistant professor of medicine, looks for the reservoirs of antibiotic-resistant organisms. Are they in the gut, on the skin, in the environment? If they find a cozy niche, do they stay for life? What turns them into a dangerous infection?
Kwon’s lab is creating “methods to detect and prevent colonization and infections. How much of the population is already colonized? Who is at greatest risk for an infection? As fast as we can develop new drugs, the microbes become resistant, so I focus on how to prevent them in the first place,” Kwon says.
“When I was a medical student, we took care of a patient with a liver transplant. She had been suffering for quite some time before the transplant, but the surgery was successful, and she was doing well,” she continues. “Later, she wound up extremely ill in the ICU with a severe multidrug-resistant infection. After going through so much to get to a transplant, she was overcome with sepsis, and there was no antibiotic available to treat her infection.” That was over 12 years ago, and it sealed Kwon’s resolve to study infectious diseases. “I’m a clinician first,” she says. “I opened the lab because I realized there were unanswered questions, and we could not help people without doing the research.”

Finding weapons
A New York Times article in December 2019 suggested that hospitals and physicians were to blame for the lack of new antibiotics since they preferred to use the older, cheaper, less effective antibiotics. The suggestion infuriated Jason Newland, MD, associate professor of pediatrics: “That is absolutely false! The cheaper antibiotics are not less effective, and we are saving the new antibiotics for the cases when we have resistant bacteria, because we don’t want to overuse them!”
Powderly adds a complication: “At places like Barnes, we do have the newest, most expensive drugs available, and we keep them in reserve. But many hospitals across the country use less expensive medications because of the way Medicare reimburses” — and that is creating a problem.
Powderly is past president of the Infectious Diseases Society of America, which has been sounding the alarm about antimicrobial resistance for nearly 20 years. In June 2021, he says, a bill they’ve advocated for was reintroduced in Congress. It would change the way the U.S. incentivizes drug development, using tax credits to make sure there is sufficient return on investment to keep companies working on new antimicrobials, keep the drugs affordable and offer “higher levels of reimbursement for resistant infections, so there isn’t a disincentive [for physicians] to use the appropriate drug.”
At the moment, “the bottom line is not there” for pharmaceutical companies, Mattar says. “You are asking for a really good new drug to be developed quickly and to be made accessible globally — but then public health experts tell companies, ‘Don’t market it; don’t overuse it!’” The usual estimate is that it costs $2.6 billion to develop a new antibiotic. “Are the R&D costs pharma claims accurate? Those are murky waters. But it is obvious there isn’t a profit incentive to develop new antibiotics the way there are incentives to develop other drugs like blood pressure, diabetes or cancer medications.” Mattar chairs an international team of experts that hopes to encourage and coordinate R&D around the world.
To address antibiotic resistance, we need more than new drugs, though. New antibiotics are necessary to treat resistant infections, but the enemy will develop resistance to them, too. To really make headway, we need new diagnostic tests, so we can swiftly detect the source of infection and pinpoint the right treatment. Some of the current tests are so slow, doctors feel obliged to prescribe antibiotics while waiting for the results. And many tests require a lab with running water and electricity, which hospitals in some countries can’t guarantee continuously.
“Hospitals and laboratories need to use molecular methods to detect the genetic material of the bacteria or virus,” Fraser says, “to speed diagnosis of the organism and detect the presence of resistance genes.” Just waiting for bacteria to grow can take days to weeks — and that’s often too long. “Also, we need rapid diagnostic tests that can be used on blood and other body fluids.”
One creative approach is to treat infections caused by antibiotic-resistant bacteria with bacteriophages — viruses that paralyze bacteria. Bacteriophages can be used in combination with antibiotics and targeted to kill certain resistant bacteria, Fraser says. “But it’s difficult and labor-intensive, and the protocol has to be developed specifically for each patient’s infection.” And after all that? Bacteria can become resistant to bacteriophages as well.
Gautam Dantas, PhD, is a professor of pathology and immunology. In his laboratory, he leads a team of experts across the fields of microbial genomics, ecology, synthetic biology and systems biology. In their search for ways to treat bacterial diseases without relying exclusively on antimicrobials, they have begun to study resistance itself: why it is so insidious and how it can be stopped.
Bacteria transfer their genes vertically to the next generation when they replicate, but they also transfer genes horizontally to unrelated organisms. That’s why the capacity for resistance can spread so easily from one person to the next, between people and animals, between groundwater and soil and people and animals. “Bacteria can copy and paste, transferring genes to a bacterium as different from them as you and I are from a banana,” Dantas explains.
The transfers of resistance genes aren’t only between pathogens, either. Back in 2008, Dantas’ lab was the first to discover that “good” bacteria, lying harmless in the soil, were transferring huge sets of their DNA — four or five or six resistance genes in a row — to microbes that cause disease. These transfers were so big, they leapfrogged evolution: “That chunk of genes can knock out five or six antibiotics at once,” Dantas says.
Luckily, the team also identified particular genes whose entire raison d’etre is to move other genes around. “They are the scissors and the glue,” he explains. “So now, when we identify a resistance gene, we look around it. If we find those genes, we know the resistance is perfectly poised to jump.” That happened recently, when they discovered a set of enzymes that could inactivate tetracycline in a new way. Although this resistance “was not yet active in pathogens, it was sitting right next to the genes that cause horizontal transfer.” In other words, it was poised to emerge — which was especially bad news because three new drugs in the tetracycline class were in the pipeline.
Before these drugs even made it to clinical trials, bacteria with resistance enzyme genes were found in humans and animals in China. “I wish I’d been wrong,” Dantas says heavily. “But luckily, in collaboration with two other labs at the university, we created new compounds that can inactivate the enzymes. These inhibitors — which are not antibiotics — can be given along with tetracycline,” preserving its potency.
The Dantas lab looks for pathogens’ Achilles heels: How do they inject toxic proteins? How can we foil that mechanism? Where is the resistance gene vulnerable? “No function is cost-free,” he points out. While a resistance gene is fighting on one front, its opponent can sneak up from another angle. “So maybe we use a combination of drugs, and while the organism is resisting one, it becomes more vulnerable to another.”
That worked with MRSA, the strain of staph infection that’s been so problematic. “We gave a combination of three generic drugs that individually do nothing against MRSA. But together, these three drugs overwhelmed its balance, and it short-circuited.”
Using the microbiome
The most promising arsenal may come from our microbiome itself.
We all walk around with trillions of microbes — bacteria, fungi, protozoa and viruses that live on our skin, in our mouths, in our guts. This collection is our microbiome, an organ as surely as the heart or lungs. It weighs about 5 pounds in a young male, its cells outnumber our own, and it has 200 times the number of genes in the human genome. Teeming and complex, the microbiome plays a critical role in our immune system. But when these microbes wind up invading other parts of our body, they can cause infection.
“There’s this phenomenon called translocation,” Powderly explains. “Bacteria from the gut cross into the bloodstream all the time. The body senses that and deals with it, eliminating them without us even knowing. But in certain circumstances — when someone is taking immunosuppressant drugs, for example — that protection breaks down, and these gut organisms can go somewhere they’re not supposed to and become pathogenic. So we need either to boost our ability to keep them where they are supposed to be or to eliminate them when they move.”
In other words, we need to make our microbiome our closest ally. “We can’t disrupt its functioning willy-nilly,” Powderly concedes. “But can we use the microbiome to enhance response, decrease virulence, even prevent infections?”

Dantas goes back to the beginning, looking at the formation of the microbiome. “We’re not born with one; it sets up early in life. Use antibiotics early, and you set it off on a bad trajectory. We’ve been able to develop an understanding of different antibiotics and how badly they might damage the gut, and we’re trying to figure out features of microbiomes that are more resilient.”
His most exciting work, though, focuses on using living organisms. All the buzz about probiotics jumped the gun, he says, because probiotics are unregulated, and what consumers can currently take are largely untargeted and could even be problematic. “Rather than go on the hunt for new probiotics, we’re using synthetic biology to genetically engineer safe organisms that do specific things.” His lab has developed two organisms, a bacterium and a yeast, that are equipped to fight pathogens the same way our immune system does.
“Let’s learn from nature,” Dantas urges. “Let’s look at the mechanisms by which phages destroy bacteria, borrow that toxin, and put it in our engineered organism.” As a weapon, it will sense, detect and destroy: “The sensors are proteins that will bind to particular pathogens. They can be detected when additional proteins turn on, maybe showing up as a fluorescent color. To destroy, they turn on an additional gene that is toxic. Now it will go destroy the pathogen.
“So we are actually working with the bugs,” he concludes, “endowing them with properties that will be beneficial to us. Rather than a military campaign, it’s our attempt at diplomacy.”