Maybe it happened after you hauled a house’s worth of boxes to and from a moving van while helping a friend move. Maybe it startled you after a seemingly innocuous fender bender. Or maybe you noticed it after spending day in and day out — for years — hunched over your laptop keyboard.
Whatever the case, it is likely that you have experienced the agony of low-back pain. One study estimates that 80 percent of the U.S. population will experience a back problem at some point in their lives. And according to the 2010 Global Burden of Disease study, low-back pain is the top contributor to disability both in the United States and globally.
Lori Setton, PhD, the Lucy and Stanley Lopata Distinguished Professor of Biomedical Engineering, has made it part of her life’s mission to help solve this problem. Much of her research focuses on developing materials for soft-tissue regeneration, which could unlock a cure for many back problems. After a successful two-decade tenure at Duke University, Setton arrived at Washington University in summer 2015 to zero in on this issue. With the help of a new set of campus collaborators, her already remarkable work has risen to a new level.
The science behind the suffering
One of the most detrimental changes that contributes to serious low-back problems — as well as many serious neck problems — is time itself. As people age, many experience degeneration of the intervertebral disc, a complex soft tissue between the vertebral bones of the spine. This degeneration can be exacerbated by demanding physical work.
Compounding the problem is that, unlike many other cells in the body, cells in the intervertebral disc stop regenerating as we get older. (It’s why your back problem might never seem to go away, but your kid’s back heals quickly.) It’s a vexing problem for scientists and patients with low-back pain.
“Basically, we have these really large structures that support our entire body — our skeletons — but they have no means to regenerate or repair themselves,” Setton explains.
What’s more, as we get older, the environment for these cells in the intervertebral disc gets increasingly inhospitable, as oxygen levels dwindle and pH levels rise in the tissue. Together, these changes make it more difficult for even the remaining cells to thrive.
For years, scientists and medical device companies tried to solve this problem by simply replacing damaged structures with artificial materials, such as polymers including polyurethane. “We thought we could just develop strong materials, inject them and solve the problem,” Setton explains.
There was one troubling detail: It didn’t work. Again and again, researchers and medical device companies failed to find a solution that improved patients’ conditions. So they returned to the drawing board.
Scientists, including Setton, are now taking a new approach. “We’re asking different questions,” she says. “‘Why are these cells dying? And how can we get a cell in this [unfavorable] environment to survive and do its job?’ We’re becoming exceptionally interested in ways of using biology to make smarter materials that will survive in this very hostile environment.”
These types of questions are opening an entirely new field of research that leans on biology, chemistry and engineering — and offers a promising road ahead.
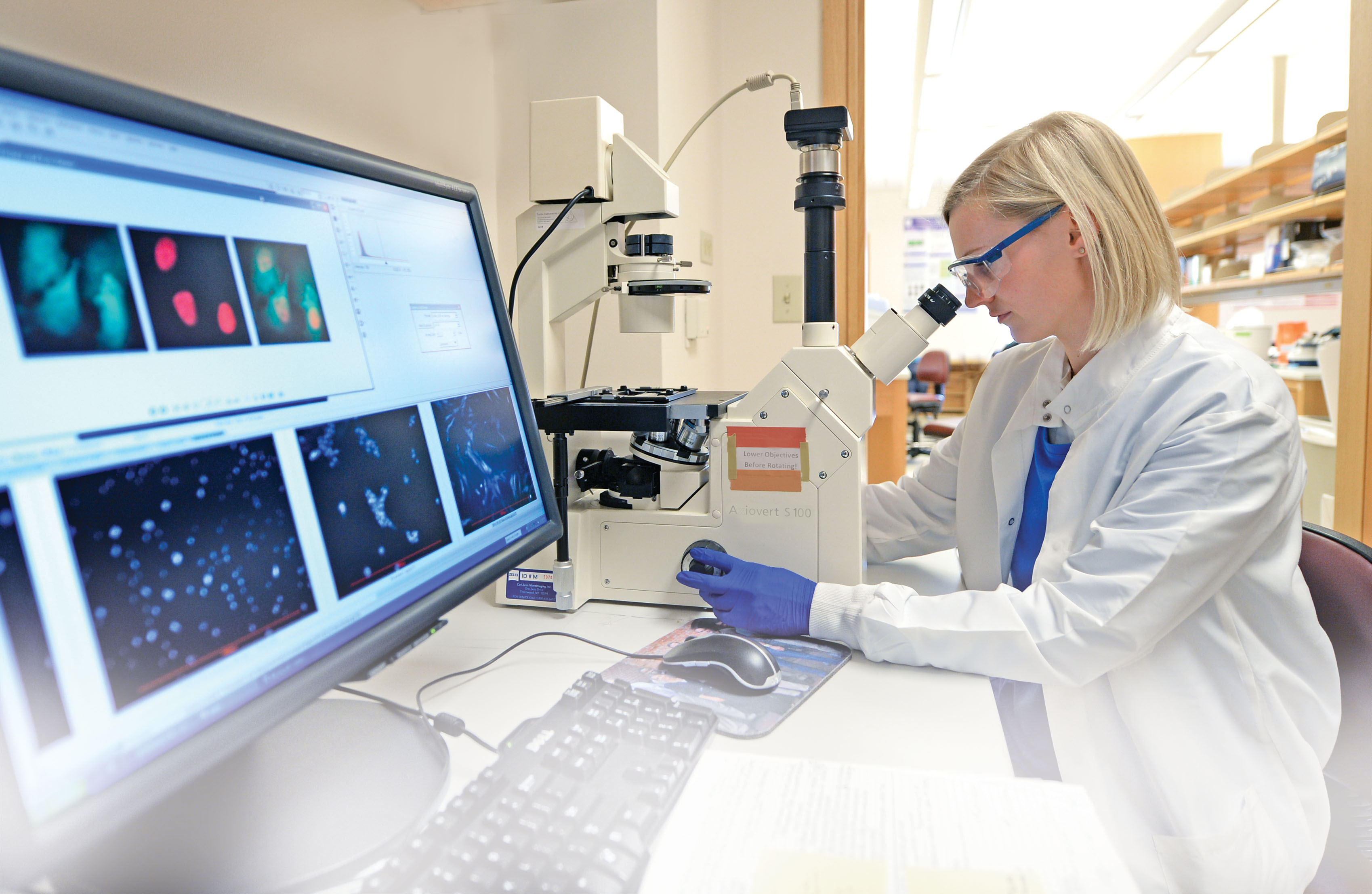
Built for a new kind of science
If there is anyone who is perfectly positioned to excel at this kind of messy, discipline-crossing research, it’s Setton. She earned a bachelor’s degree in mechanical and aerospace engineering from Princeton, then followed it up with master’s and doctoral degrees in mechanical engineering and biomechanics from Columbia University. It’s a background that gives her special insight into how our bodies work — and how they can be fixed when they break down.
Now she uses her expertise to understand why cells in our intervertebral disc regenerate when we’re young, and what they lose over time that prevents them from regenerating as we age.
During the past few years, for example, she and others have learned that certain proteins within the disc shift over time. One protein, called laminin, appears to be particularly important in early development: It exists in juvenile structures, but it’s absent in adults.
“We started to ask questions about the effect of reintroducing laminin,” Setton says. “So we built two- and three-dimensional polymers that are capable of presenting laminin back to these cells when we grow them in culture,” she says.
The process of building these structures — known as cellular engineering — is complex. First, she and her team take nonfunctioning cells from human subjects. Then they grow them in tissue culture wells (similar to petri dishes) that have been modified with specific proteins and polymers known as biomaterials. Once she and her team have grown the cells and introduced the laminin, they study them to see if the cells are reverting to juvenile behavior.
“It’s been pretty exciting to see these cells [regain] a lot of the behaviors of the juvenile cells. They become biosynthetically active, and they do a good job repairing [themselves] in this environment.”
—Lori Setton
The results so far have been encouraging. “It’s been pretty exciting to see these cells [regain] a lot of the behaviors of the juvenile cells,” Setton says. “They become biosynthetically active, and they do a good job repairing [themselves] in this environment.”
Although it’s a long way from petri dish to clinical solution, it appears to be one very big step in the right direction.
“Steps like these are possible because of the combination of skills that Setton brings to the table,” says Aaron Bobick, dean of Washington University’s School of Engineering & Applied Science. After all, the real-world problems we face have never respected the artificial boundaries that humans place between engineering and medicine, biology and chemistry.
“Nobody told the tissue in your spine that it needed a mechanical solution or a biological solution or a chemical solution,” says Bobick, also the James M. McKelvey Professor. “It’s at this interdisciplinary boundary that the advancements happen, and a lot of what goes on in biomedical engineering requires those multidisciplinary efforts. Lori brings that all together in her laboratory.”
Collaboration fuels success
Though Setton brings a wealth of scientific tools to her work, perhaps her most remarkable skill is her ability to collaborate with other researchers on projects that leverage everyone’s expertise in unique ways.
For example, before Setton had officially started at her position at WashU, she tracked down Don Elbert, PhD, associate professor of biomedical engineering, whose work on biomaterials she had long admired from afar. The two quickly saw how his work on polyethylene glycol could link up with her own work in biomaterials. In a matter of weeks, they pulled together an application for an NIH grant.
“[She] is great at recognizing where other people have expertise that’s complementary to hers. But even better, she’s a very generous collaborator. She shares both the credit and the accolades.”
—Don Elbert
In December 2015, the grant that the pair developed scored in the top 1 percent of all applications for the cycle, and they were recently awarded $1.2 million in NIH funding to further develop their ideas and innovations.
The two will also collaborate with Munish Gupta, MD, the Mildred B. Simon Distinguished Professor of Orthopedic Surgery and chief of Pediatric and Adult Spinal Surgery in the School of Medicine. Gupta, who arrived at WashU in summer 2015 as well, brings deep expertise in complex spinal deformities, and he is eager to work on these types of collaborative projects.
Elbert says that Setton makes for an ideal partner in such projects. “[She] is great at recognizing where other people have expertise that’s complementary to hers,” he says. “But even better, she’s a very generous collaborator. She shares both the credit and the accolades, and that matters.”
Setton’s generosity, paired with her holistic approach to mentorship, was what helped her convince MD/PhD student Elizabeth Leimer to follow her from Duke to WashU. Leimer, who is studying the proteins and receptors that contribute to painful responses in a degenerated disc, says that though it would have been logistically easier for her to stay at Duke, she couldn’t pass up the chance to finish her work with Setton.

Leimer specifically appreciates how Setton has helped her design projects that will allow her to have the biggest scientific impact in the limited time she has as an MD/PhD student. Setton provides constructive feedback to students like Leimer, who often have more interesting scientific opportunities than time.
“Sometimes people approach me [because of my scientific knowledge] and ask to work together on a side project,” Leimer says. “If I think it would be an interesting project, I tell [Setton] about it, and she’ll say, ‘Well, that would be great. But how are you going to get this done? How is that going to help you meet your goals?’ I like to run everything by her as a reality check.”
Indeed, Setton has long been praised for her mentorship. And, in 2004, Duke Graduate School honored her with the Dean’s Award for Excellence in Mentoring. But Setton says her best advice will be valuable to students long after they leave her lab. “I want to help them build a lifetime approach to asking, ‘Where am I now and where am I going to go next? What do I need to get there?’” Setton says. “I want to prepare them to succeed in any number of settings.”
Leimer also has been grateful to have a female mentor. “I’ve had many male mentors, but I knew Professor Setton could bring a different perspective, including what it means to be a successful woman in science,” Leimer says.
“Recently, I’ve been focused on building mentoring networks with more senior women. When you have a big cohort of successful senior female leaders, then you’re in a much better place to prepare the next generation of female students to succeed.”
—Lori Setton
Setton recognizes that she can play a particularly valuable role for women, which is a responsibility she takes seriously. “Even though fully half of our students in biomedical engineering are women — the pipeline is deep — we still don’t see women rising to the level of leadership,” she says. “Recently, I’ve been focused on building mentoring networks with more senior women. When you have a big cohort of successful senior female leaders, then you’re in a much better place to prepare the next generation of female students to succeed.”
Setton will have another significant opportunity to guide the field now that she’s the new president of the Biomedical Engineering Society, the premier professional society for the discipline. In addition to accrediting degrees and overseeing the process of education, the organization connects industry and academics and runs career development programming. “The organization defines new directions — where the field is going,” Setton says. “Because this is a really young and dynamic field, it’s an exciting time to be president.”
The road ahead
As Setton looks to the future, she sees many opportunities to strengthen her research, find additional collaborators at WashU, and build better opportunities for the students in her lab and for women scientists more generally.
She’s particularly eager to build collaborative working relationships with many colleagues she’s known through professional meetings and networks for years, and who now work just down the hall. Her plate is full, but she’s determined to make the most of it. “There are things that I can do now that I could never have dreamed about doing before,” she says.
The opportunities are enormous. And Lori Setton is just getting started.
Erin Peterson is a freelance writer and founder of Capstone Communications based in Minneapolis.
